Exotic Pet Trade as a Cause of Biological Invasions: The Case of Tree Squirrels of the Genus Callosciurus
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Genus Callosciurus
3. Distribution of IAS and Pathways of Introduction
3.1. Native Range
3.2. Areas of Introduction
3.3. Pathways of Introduction
4. Origin and the Challenge of Species Identification
5. Early Detection and Effective Monitoring of IAS
6. The Establishment and Spread of IAS
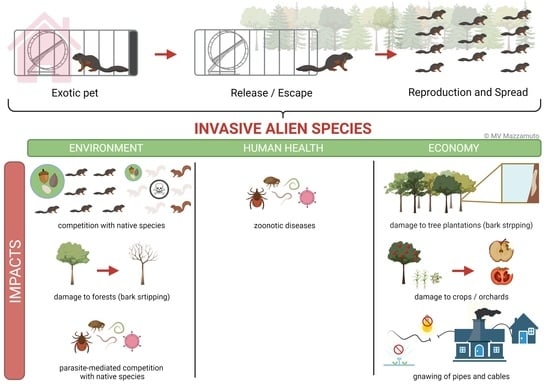
7. Impacts of IAS on Environment, Economy and Human Health
7.1. Harm to Native Species
7.2. Parasites, Zoonosis and Human Health
7.2.1. Macroparasites
7.2.2. Microparasites
7.3. Harm to Human Activities, Properties and Economic Impact
8. Management and Regulations: Failures and Successes
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APPA. Annual Report; American Pet Product Associetion: Stamphord, CT, USA, 2021. [Google Scholar]
- FEDIAF. Annual Report; The European Pet Food Industry: Bruxelles, Belgium, 2021. [Google Scholar]
- Novák, J.; Kalous, L.; Patoka, J. Modern Ornamental Aquaculture in Europe: Early History of Freshwater Fish Imports. Rev. Aquac. 2020, 12, 2042–2060. [Google Scholar] [CrossRef]
- Ding, J.; Mack, R.N.; Lu, P.; Ren, M.; Huang, H. China’s Booming Economy Is Sparking and Accelerating Biological Invasions. BioScience 2008, 58, 317–324. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Lima, J.R.D.F.; Araujo, H.F.P. The Live Bird Trade in Brazil and Its Conservation Implications: An Overview. Bird Conserv. Int. 2013, 23, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Lockwood, J.L.; Welbourne, D.J.; Romagosa, C.M.; Cassey, P.; Mandrak, N.E.; Strecker, A.; Leung, B.; Stringham, O.C.; Udell, B.; Episcopio-Sturgeon, D.J.; et al. When Pets Become Pests: The Role of the Exotic Pet Trade in Producing Invasive Vertebrate Animals. Front. Ecol. Environ. 2019, 17, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Scheffers, B.R.; Oliveira, B.F.; Lamb, I.; Edwards, D.P. Global Wildlife Trade across the Tree of Life. Science 2019, 366, 71–76. [Google Scholar] [CrossRef]
- Springborn, M.R.; Keller, R.P.; Elwood, S.; Romagosa, C.M.; Zambrana-Torrelio, C.; Daszak, P. Integrating Invasion and Disease in the Risk Assessment of Live Bird Trade. Divers. Distrib. 2015, 21, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Collis, A.H.; Fenili, R.N. The Modern U.S. Reptile Industry; Georgetown Economic Services, LLC: Washington, DC, USA, 2011; p. 93. [Google Scholar]
- Kopecký, O.; Kalous, L.; Patoka, J. Establishment Risk from Pet-Trade Freshwater Turtles in the European Union. Knowl. Manag. Aquat. Ecosyst. 2013, 410, 2. [Google Scholar] [CrossRef] [Green Version]
- Hulme, P.E. Invasion Pathways at a Crossroad: Policy and Research Challenges for Managing Alien Species Introductions. J. Appl. Ecol. 2015, 52, 1418–1424. [Google Scholar] [CrossRef]
- Gippet, J.M.W.; Bertelsmeier, C. Invasiveness Is Linked to Greater Commercial Success in the Global Pet Trade. Proc. Natl. Acad. Sci. USA 2021, 118, e2016337118. [Google Scholar] [CrossRef]
- Rosa, C.A.; de Almeida Curi, N.H.; Puertas, F.; Passamani, M. Alien Terrestrial Mammals in Brazil: Current Status and Management. Biol. Invasions 2017, 19, 2101–2123. [Google Scholar] [CrossRef]
- Krysko, K.L.; Burgess, J.P.; Rochford, M.R.; Gillette, C.R.; Cueva, D.; Enge, K.M.; Somma, L.A.; Stabile, J.L.; Smith, D.C.; Wasilewski, J.A.; et al. Verified Non-Indigenous Amphibians and Reptiles in Florida from 1863 through 2010: Outlining the Invasion Process and Identifying Invasion Pathways and Stages. Zootaxa 2011, 3028, 1–64. [Google Scholar] [CrossRef] [Green Version]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, D.; Nentwig, W.; Olenin, S.; Panov, V.; Pergl, J.; et al. Grasping at the Routes of Biological Invasions: A Framework for Integrating Pathways into Policy. J. Appl. Ecol. 2008, 45, 403–414. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing Pathways of Introduction of Marine Aliens. Ocean. Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Saul, W.-C.; Roy, H.E.; Booy, O.; Carnevali, L.; Chen, H.-J.; Genovesi, P.; Harrower, C.A.; Hulme, P.E.; Pagad, S.; Pergl, J.; et al. Assessing Patterns in Introduction Pathways of Alien Species by Linking Major Invasion Data Bases. J. Appl. Ecol. 2017, 54, 657–669. [Google Scholar] [CrossRef]
- Duggan, I.C.; Rixon, C.A.M.; MacIsaac, H.J. Popularity and Propagule Pressure: Determinants of Introduction and Establishment of Aquarium Fish. Biol. Invasions 2006, 8, 377–382. [Google Scholar] [CrossRef]
- Keller, R.P.; Geist, J.; Jeschke, J.M.; Kühn, I. Invasive Species in Europe: Ecology, Status, and Policy. Environ. Sci. Eur. 2011, 23, 23. [Google Scholar] [CrossRef] [Green Version]
- Stringham, O.C.; Lockwood, J.L. Pet Problems: Biological and Economic Factors That Influence the Release of Alien Reptiles and Amphibians by Pet Owners. J. Appl. Ecol. 2018, 55, 2632–2640. [Google Scholar] [CrossRef]
- Hulme, P.E. New Law Risks Release of Invasive Species. Nature 2015, 517, 21. [Google Scholar] [CrossRef]
- European Environment Agency. The Impacts of Invasive Alien Species in Europe; Publications Office of the European Union: Luxembourg, 2013; ISBN 978-92-9213-345-0. [Google Scholar]
- Marbuah, G.; Gren, I.-M.; McKie, B. Economics of Harmful Invasive Species: A Review. Diversity 2014, 6, 500–523. [Google Scholar] [CrossRef] [Green Version]
- Mori, E.; Meini, S.; Strubbe, D.; Ancillotto, L.; Sposimo, P.; Menchetti, M. Do alien free-ranging birds affect human health? A global summary of known zoonoses. In Invasive Species and Human Health; CAB: Wallingford, Oxfordshire, UK; Boston, MA, USA, 2018; ISBN 978-1-78639-098-1. [Google Scholar]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the Environmental and Economic Costs Associated with Alien-Invasive Species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Kraus, F. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; ISBN 978-1-4020-8946-6. [Google Scholar]
- Nogueira, D.M.; Ferreira, A.M.R.; Goldschmidt, B.; Pissinatti, A.; Carelli, J.B.; Verona, C.E. Cytogenetic Study in Natural Hybrids of Callithrix (Callitrichidae: Primates) in the Atlantic Forest of the State of Rio de Janeiro, Brazil. Iheringia Sér. Zool. 2011, 101, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Duscher, T.; Hodžić, A.; Glawischnig, W.; Duscher, G.G. The Raccoon Dog (Nyctereutes procyonoides) and the Raccoon (Procyon lotor)—Their Role and Impact of Maintaining and Transmitting Zoonotic Diseases in Austria, Central Europe. Parasitol. Res. 2017, 116, 1411–1416. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Beck, B.; García, F.J.; Gortázar, C. Raccoons in Europe: Disease Hazards Due to the Establishment of an Invasive Species. Eur. J. Wildl. Res. 2012, 58, 5–15. [Google Scholar] [CrossRef]
- Bertolino, S. Introduction of the American Grey Squirrel (Sciurus carolinensis) in Europe: A Case Study in Biological Invasion. Curr. Sci. 2008, 95, 903–906. [Google Scholar]
- Derbridge, J.; Pepper, H.; Koprowski, J.L. Economic Damage by Invasive Grey Squirrels in Europe. In The Grey Squirrel: Ecology and Management of an Invasive Species in Europe; Shuttleworth, C.M., Lurz, P.W., Gurnell, J., Eds.; European Squirrel Initiative: Woodbridge, UK, 2016; pp. 393–405. [Google Scholar]
- Bertolino, S. Animal Trade and Non-Indigenous Species Introduction: The World-Wide Spread of Squirrels. Divers. Distrib. 2009, 15, 701–708. [Google Scholar] [CrossRef]
- Jeschke, J.M.; Strayer, D.L. Invasion Success of Vertebrates in Europe and North America. Proc. Natl. Acad. Sci. USA 2005, 102, 7198–7202. [Google Scholar] [CrossRef] [Green Version]
- Long, J.L. Introduced Mammals of the World. Their History, Distribution and Influence; CSIRO Publishing: Wallingford, UK, 2003. [Google Scholar]
- Jessen, R.R.; Merrick, M.J.; Koprowski, J.L.; Ramirez, O. Presence of Guayaquil Squirrels on the Central Coast of Peru: An Apparent Introduction. Mammalia 2010, 74, 443–444. [Google Scholar] [CrossRef]
- Palmer, G.H.; Koprowski, J.; Pernas, T. Tree Squirrels as Invasive Species: Conservation and Management Implications. In Managing Vertebrate Invasive Species: Proceeding of an International Symposium, Fagerstone; Witmer, G.W., Pitt, W.C., Fagerstone, K.A., Eds.; USDA/APHIS/WS, National Wildlife Research Center: Fort Collins, CO, USA, 2007. [Google Scholar]
- Davis, R.; Brown, D.E. Documentation of the Transplanting of Abert’s Squirrels. Southwest. Nat. 1988, 33, 490–492. [Google Scholar] [CrossRef]
- Martinoli, A.; Bertolino, S.; Preatoni, D.G.; Balduzzi, A.; Marsan, A.; Genovesi, P.; Tosi, G.; Wauters, L.A. Headcount 2010: The Multiplication of the Grey Squirrel Populations Introduced to Italy. Hystrix Ital. J. Mammal. 2010, 21, 127–136. [Google Scholar]
- Shuttleworth, C.M.; Lurz, P.W.W.; Gurnell, J. The Grey Squirrel: Ecology & Management of an Invasive Species in Europe; European Squirrel Initiative: Woodbridge, UK, 2016. [Google Scholar]
- Wauters, L.A.; Tosi, G.; Gurnell, J. Interspecific Competition in Tree Squirrels: Do Introduced Grey Squirrels (Sciurus carolinensis) Deplete Tree Seeds Hoarded by Red Squirrels (S. vulgaris)? Behav. Ecol. Sociobiol. 2002, 51, 360–367. [Google Scholar] [CrossRef]
- Wauters, L.A.; Gurnell, J.; Martinoli, A.; Tosi, G. Interspecific Competition between Native Eurasian Red Squirrels and Alien Grey Squirrels: Does Resource Partitioning Occur? Behav. Ecol. Sociobiol. 2002, 52, 332–341. [Google Scholar] [CrossRef]
- Gurnell, J.; Wauters, L.A.; Lurz, P.W.W.; Tosi, G. Alien Species and Interspecific Competition: Effects of Introduced Eastern Grey Squirrels on Red Squirrel Population Dynamics. J. Anim. Ecol. 2004, 73, 26–35. [Google Scholar] [CrossRef]
- Romeo, C.; Piscitelli, A.P.; Santicchia, F.; Martinoli, A.; Ferrari, N.; Wauters, L.A. Invading Parasites: Spillover of an Alien Nematode Reduces Survival in a Native Species. Biol. Invasions 2021. [Google Scholar] [CrossRef]
- Lioy, S.; Marsan, A.; Balduzzi, A.; Wauters, L.A.; Martinoli, A.; Bertolino, S. The Management of the Introduced Grey Squirrel Seen through the Eyes of the Media. Biol. Invasions 2019, 21, 3723–3733. [Google Scholar] [CrossRef]
- Bertolino, S.; Lurz, P.W.W. Callosciurus Squirrels: Worldwide Introductions, Ecological Impacts and Recommendations to Prevent the Establishment of New Invasive Populations. Mammal. Rev. 2013, 43, 22–33. [Google Scholar] [CrossRef]
- Thorington, R.W.J.; Koprowski, J.L.; Steele, M.A.; Whatton, J.F. Squirrels of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2012; ISBN 978-1-4214-0469-1. [Google Scholar]
- Moore, J.C.; Tate, G.H.H. A Study of the Diurnal Squirrels: Sciurinae, of the Indian and Indochinese Subregions. In Fieldiana, Zoology; Chicago Natural History Museum: Chicago, IL, USA, 1965; Volume 48, pp. 1–351. [Google Scholar]
- Corbet, G.B.; Hill, J.E. The Mammals of the Indomalayan Region: A Systematic Review; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Nguyen, S.T.; Oshida, T.; Dang, P.H.; Bui, H.T.; Motokawa, M. A New Species of Squirrel (Sciuridae: Callosciurus) from an Isolated Island off the Indochina Peninsula in Southern Vietnam. J. Mammal. 2018, 99, 813–825. [Google Scholar] [CrossRef]
- Thorington, R., Jr.; Hoffmann, R.S. Family Sciuridae. In Mammal Species of the World: A Taxonomic and Geographic Reference; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 754–818. [Google Scholar]
- Loy, A.; Aloise, G.; Ancillotto, L.; Angelici, F.M.; Bertolino, S.; Capizzi, D.; Castiglia, R.; Colangelo, P.; Contoli, L.; Cozzi, B.; et al. Mammals of Italy: An Annotated Checklist. Hystrix Ital. J. Mammal. 2019, 30, 87–106. [Google Scholar] [CrossRef]
- Li, S.; Feng, Q.; Wang, Y.X. A New Subspecies in Callosciurus erythraeus (Rodentia, Sciurdae). Acta Zootaxonomica Sin. 2006, 31, 675–682. [Google Scholar]
- Ellerman, J.R.; Morrison-Scott, T.C.S. Checklist of Palaearctic and Indian Mammals, 1758 to 1946; Trustees of the British Museum: London, UK, 1951. [Google Scholar]
- Gabrielli, M.; Cardoso, Y.P.; Benitez, V.; Gozzi, A.C.; Guichón, M.L.; Lizarralde, M.S. Genetic Characterization of Callosciurus (Rodentia: Sciuridae) Asiatic Squirrels Introduced in Argentina. Ital. J. Zool. 2014, 81, 328–343. [Google Scholar] [CrossRef] [Green Version]
- Mazzamuto, M.V.; Galimberti, A.; Cremonesi, G.; Pisanu, B.; Chapuis, J.-L.; Stuyck, J.; Amori, G.; Su, H.; Aloise, G.; Preatoni, D.G.; et al. Preventing Species Invasion: A Role for Integrative Taxonomy? Integr. Zool. 2016, 11, 214–228. [Google Scholar] [CrossRef]
- Balakirev, A.E.; Rozhnov, V.V. Taxonomic Revision of Beautiful Squirrels (Callosciurus, Rodentia: Sciuridae) from the Callosciurus erythraeus/Finlaysonii Complex and Their Distribution in Eastern Indochina. Raffles Bull. Zool. 2019, 67, 459489. [Google Scholar] [CrossRef]
- Lurz, P.W.W.; Hayssen, V.; Geissler, K.; Bertolino, S. Callosciurus erythraeus (Rodentia: Sciuridae). Mamm. Species 2013, 902, 60–74. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, J.W. Callosciurus finlaysonii. IUCN Red List Threat. Species 2017, e.T3596A22254494. [Google Scholar] [CrossRef]
- Duckworth, J.W.; Robichaud, W.G. Yellow-Bellied Weasel Mustela Kathiah Sightings in Phongsaly Province, Laos, with Notes on the Species’ Range in South-East Asia, and Recent Records of Other Small Carnivores in the Province. Small Carniv. Conserv. 2005, 33, 17–20. [Google Scholar]
- Timmins, R.J.; Duckworth, J.W. Diurnal Squirrels (Mammalia Rodentia Sciuridae) in Lao PDR: Distribution, Status and Conservation. Trop. Zool. 2008, 21, 11–56. [Google Scholar]
- Adriaens, T.; Baert, K.; Breyne, P.; Casaer, J.; Devisscher, S.; Onkelinx, T.; Pieters, S.; Stuyck, J. Successful Eradication of a Suburban Pallas’s Squirrel Callosciurus erythraeus (Pallas 1779) (Rodentia, Sciuridae) Population in Flanders (Northern Belgium). Biol. Invasions 2015, 17, 2517–2526. [Google Scholar] [CrossRef]
- La Haye, M. Pallas’ squirrel eradication in the Netherlands. Case study in: Invasive Alien Species Colonisation Prevention: Your guide to early detection and rapid response. In Invasive Alien Species Colonisation Prevention: Your Guide to Early Detection and Rapid Response; The Royal Society of Wildlife Trusts: Newark, UK, 2020; pp. 145–153. ISBN 978-1-5272-5405-3. [Google Scholar]
- Tamura, N.; Kasahi, T.; Kaneda, M.; Mitarai, N.; Shigeta, M.; Shigeta, Y.; Yamasaki, F.; Morisaki, M.; Tsuda, T.; Ono, S.; et al. Sound Playback Surveys to Reveal the Distribution of Invasive Alien Pallas’s Squirrels, Callosciurus erythraeus. Mammal. Study 2013, 38, 97–103. [Google Scholar] [CrossRef]
- Yasuda, M. The Pallas’s squirrel has likely established a new feral population on Mt. Kirishima, on the border between Miyazaki and Kagoshima Prefectures, Kyushu, Southwestern Japan. Sciurid. Inf. 2013, 30, 15. [Google Scholar]
- Benitez, V.; Gozzi, A.C.; Borgnia, M.; Almada Chavez, S.; Messetta, M.L.; Clos Clos, G.; Guichón, M.L. La Ardilla de Vientre Rojo En Argentina: Investigación y Educación, Puntos Clave Para El Manejo de Una Especie Invasora. In Invasiones Biológicas: Avances 2009; GEIB Grupo Especialista en Invasiones Biológicas: León, Spain, 2010; pp. 255–260. [Google Scholar]
- Guichón, M.L.; Benitez, V.V.; Gozzi, A.C.; Hertzriken, M.; Borgnia, M. From a Lag in Vector Activity to a Constant Increase of Translocations: Invasion of Callosciurus Squirrels in Argentina. Biol. Invasions 2015, 17, 2597–2604. [Google Scholar] [CrossRef]
- Guichón, M.L.; Borgnia, M.; Gozzi, A.C.; Benitez, V.V. Invasion Pathways and Lag Times in the Spread of Callosciurus erythraeus Introduced into Argentina. J. Nat. Conserv. 2020, 58, 125899. [Google Scholar] [CrossRef]
- Chapuis, J.-L.; Gerriet, O.; Losinger-Chabod, I.; Pisanu, B. Gestion d’espèces exotiques envahissantes: Le cas des ecureuils en France. Faune Sauvage (ONCFS), 2018; n°321, 45–51. [Google Scholar]
- Miyamoto, A.; Tamura, N.; Sugimura, K.; Yamada, F. Predicting Habitat Distribution of the Alien Formosan Squirrel Using Logistic Regression Model. Glob. Environ. Res. 2004, 8, 13–22. [Google Scholar]
- Ho, C.-Y. The Ecology of Exotic Squirrels (Sciuridae) in Hong Kong, with Special Reference to Caliosciurus erythraeus Thai (Kloss). Bachelor’s Thesis, University of Hong Kong, Hong Kong, 1994. [Google Scholar]
- Dozières, A.; Pisanu, B.; Kamenova, S.; Bastelica, F.; Gerriet, O.; Chapuis, J.-L. Range Expansion of Pallas’s Squirrel (Callosciurus erythraeus) Introduced in Southern France: Habitat Suitability and Space Use. Mamm. Biol. Z. Säugetierkunde 2015, 80, 518–526. [Google Scholar] [CrossRef]
- Aprile, G.; Chicco, D. Nueva especie exotica de mamifero en la Argentina: La ardilla de vientre rojo. Mastozool. Neotrop. 1999, 6, 7–14. [Google Scholar]
- Dijkstra, V.; Dekker, J. Risico-Assessment Uitheemse Eekhoorns. In Opdracht van Commissie; Invasieve Exoten, LNV: Arnhem, The Netherlands, 2008. [Google Scholar]
- Mazzamuto, M.V.; Su, H.-J.; Guidarelli, G.; Preatoni, D.; Russo, L.F.; Loy, A.; Martinoli, A. Mandible Morphology as a Tool to Investigate Origin, Adaptation and Stress in Invasive Alien Species: First Insights into Callosciurus erythraeus (Rodentia: Sciuridae) in Europe. Eur. Zool. J. 2021, 88, 782–795. [Google Scholar] [CrossRef]
- Bertolino, S.; Currado, I.; Mazzoglio, P.J. Finlayson’s (Variable) Squirrel Callosciurus finlaysoni in Italy. In Mammalia; Muséum national d’Histoire naturelle: Paris, France, 1999; Volume 63, pp. 522–525. [Google Scholar]
- Aloise, G.; Bertolino, S. Free-Ranging Population of the Finlayson’s Squirrel Callosciurus finlaysonii (Horsfield, 1824) (Rodentia, Sciuridae) in South Italy. Hystrix Ital. J. Mammal. 2005, 16, 70–74. [Google Scholar]
- Oshida, T.; Torii, H.; Lin, L.-K.; Lee, J.-K.; Chen, Y.-J.; Endo, H.; Sasaki, M. A Preliminary Study on Origin of Callosciurus Squirrels Introduced into Japan. Mammal. Study 2007, 32, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Kuramoto, T.; Torii, H.; Ikeda, H.; Endo, H.; Rerkamnuaychoke, W.; Oshida, T. Mitochondria DNA Sequences of Finlayson’s Squirrel Found in Hamamatsu, Shizuoka Prefecture, Japan. Mammal. Study 2012, 37, 63–67. [Google Scholar] [CrossRef]
- Pisanu, B.; Obolenskaya, E.V.; Baudry, E.; Lissovsky, A.A.; Chapuis, J.-L. Narrow Phylogeographic Origin of Five Introduced Populations of the Siberian Chipmunk Tamias (Eutamias) Sibiricus (Laxmann, 1769) (Rodentia: Sciuridae) Established in France. Biol. Invasions 2013, 15, 1201–1207. [Google Scholar] [CrossRef]
- Signorile, A.L.; Wang, J.; Lurz, P.W.W.; Bertolino, S.; Carbone, C.; Reuman, D.C. Do Founder Size, Genetic Diversity and Structure Influence Rates of Expansion of North American Grey Squirrels in Europe? Divers. Distrib. 2014, 20, 918–930. [Google Scholar] [CrossRef]
- Simberloff, D.; Dayan, T.; Jones, C.; Ogura, G. Character Displacement and Release in the Small Indian Mongoose, Herpestes Javanicus. Ecology 2000, 81, 2086–2099. [Google Scholar] [CrossRef] [Green Version]
- Melero, Y.; Palazón, S.; Gosàlbez, J. Morphological Adaptation of an Invasive American Mink Population in Mediterranean Areas of Spain. Acta Zool. 2007, 89, 47–51. [Google Scholar] [CrossRef]
- Firmat, C.; Schliewen, U.K.; Losseau, M.; Alibert, P. Body Shape Differentiation at Global and Local Geographic Scales in the Invasive Cichlid Oreochromis Mossambicus. Biol. J. Linn. Soc. 2012, 105, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Oshida, T.; Dang, C.N.; Nguyen, S.T.; Nguyen, N.X.; Endo, H.; Kimura, J.; Sasaki, M.; Hayashida, A.; Takano, A.; Koyabu, D.; et al. Phylogenetic Position of Callosciurus erythraeus Griseimanus from Vietnam in the Genus Callosciurus. Mammal. Study 2013, 38, 41–47. [Google Scholar] [CrossRef]
- Boonkhaw, P.; Prayoon, U.; Kanchanasaka, B.; Hayashi, F.; Tamura, N. Colour Polymorphism and Genetic Relationships among Twelve Subspecies of Callosciurus finlaysonii in Thailand. Mamm. Biol. 2017, 85, 6–13. [Google Scholar] [CrossRef]
- Tamura, N. Pallas’s squirrel. In Handbook of Alien Species in Japan; Chijin-Shokan: Tokyo, Japan, 2002; p. 66. [Google Scholar]
- Abe, H. A Guide to the Mammals of Japan. Tokai University Press: Tokai, Japan, 2005. [Google Scholar]
- Ikeda, H.; Yasuda, M.; Sakanashi, M.; Oshida, T. Origin of Callosciurus erythraeus Introduced into the Uto Peninsula, Kumamoto, Japan, Inferred from Mitochondrial DNA Analysis. Mammal. Study 2011, 36, 61–65. [Google Scholar] [CrossRef]
- Tamura, N.; Boonkhaw, P.; Prayoon, U.; Kanchanasaka, B.; Hayashi, F. Mating Calls Are a Sensitive Indicator of Phylogenetic Relationships in Tropical Tree Squirrels (Callosciurus Spp.). Mamm. Biol. 2018, 93, 198–206. [Google Scholar] [CrossRef]
- Simberloff, D. How Much Information on Population Biology Is Needed to Manage Introduced Species? Conserv. Biol. 2003, 17, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of Biological Invasions: What’s What and the Way Forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Tamura, N. Snake-Directed Mobbing by the Formosan Squirrel Callosciurus erythraeus thaiwanensis. Behav. Ecol. Sociobiol. 1989, 24, 175–180. [Google Scholar] [CrossRef]
- Tamura, N.; Yong, H.-S. Vocalizations in Response to Predators in Three Species of Malaysian Callosciurus (Sciuridae). J. Mammal. 1993, 74, 703–714. [Google Scholar] [CrossRef]
- Bertolino, S.; Wauters, L.A.; Pizzul, A.; Molinari, A.; Lurz, P.; Tosi, G. A General Approach of Using Hair-Tubes to Monitor the European Red Squirrel: A Method Applicable at Regional and National Scales. Mamm. Biol. Z. Säugetierkunde 2009, 74, 210–219. [Google Scholar] [CrossRef]
- Goldstein, E.A.; Lawton, C.; Sheehy, E.; Butler, F. Locating Species Range Frontiers: A Cost and Efficiency Comparison of Citizen Science and Hair-Tube Survey Methods for Use in Tracking an Invasive Squirrel. Wildl. Res. 2014, 41, 64. [Google Scholar] [CrossRef]
- Gurnell, J.; Lurz, P.W.W.; Shirley, M.D.F.; Cartmel, S.; Garson, P.J.; Magris, L.; Steele, J. Monitoring Red Squirrels Sciurus vulgaris and Grey Squirrels Sciurus carolinensis in Britain. Mammal. Rev. 2004, 34, 51–74. [Google Scholar] [CrossRef]
- Fasola, L.; Bello, M.; Guichón, M.L. Uso de trampas de pelo y caracterización de los pelos de la ardilla de vientre rojo Callosciurus erythraeus. Mastozoología Neotrop. 2012, 12, 9–17. [Google Scholar]
- Ancillotto, L.; Notomista, T.; Mori, E.; Bertolino, S.; Russo, D. Assessment of Detection Methods and Vegetation Associations for Introduced Finlayson’s Squirrels (Callosciurus finlaysonii) in Italy. Environ. Manag. 2018, 61, 875–883. [Google Scholar] [CrossRef]
- Benitez, V.V.; Almada Chavez, S.; Gozzi, A.C.; Messetta, M.L.; Guichón, M.L. Invasion Status of Asiatic Red-Bellied Squirrels in Argentina. Mamm. Biol. Z. Säugetierkunde 2013, 78, 164–170. [Google Scholar] [CrossRef]
- Borgnia, M.; de Bargas, S.; Valverde, A.; Forte, S.; Roldán, S. Invasiones biológicas: El arribo de la ardilla de vientre rojo (Callosciurus erytrhraeus) a la Ciudad Autónoma de Buenos Aires. Agron. Ambiente 2019, 39, 119–130. [Google Scholar]
- Tamura, N.; Hayashi, F.; Miyashita, K. Spacing and Kinship in the Formosan Squirrel Living in Different Habitats. Oecologia 1989, 79, 344–352. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Bisi, F.; Wauters, L.A.; Preatoni, D.G.; Martinoli, A. Interspecific Competition between Alien Pallas’s Squirrels and Eurasian Red Squirrels Reduces Density of the Native Species. Biol. Invasions 2017, 19, 723–735. [Google Scholar] [CrossRef]
- Santicchia, F.; Romeo, C.; Grilli, G.; Vezzoso, S.; Wauters, L.A.; Mazzamuto, M.V.; Martinoli, A.; Ferrari, N. The Use of Uterine Scars to Explore Fecundity Levels in Invasive Alien Tree Squirrels. Hystrix Ital. J. Mammal. 2015, 26, 95–101. [Google Scholar]
- Guichón, M.L.; Bello, M.; Fasola, L. Expansión poblacional de una especie introducida en la Argentina: La ardilla de vientre rojo Callosciurus erythraeus. Mastozool. Neotrop. 2005, 12, 189–197. [Google Scholar]
- Bridgman, L.J.; Benitez, V.V.; Graña Grilli, M.; Mufato, N.; Acosta, D.; Guichón, M.L. Short Perceptual Range and yet Successful Invasion of a Fragmented Landscape: The Case of the Red-Bellied Tree Squirrel (Callosciurus erythraeus) in Argentina. Landsc. Ecol. 2012, 27, 633–640. [Google Scholar] [CrossRef]
- Wauters, L.; Amori, G.; Aloise, G.; Spartaco, G.; Agnelli, P.; Galimberti, A.; Casiraghi, M.; Preatoni, D.; Martinoli, A. New Endemic Mammal Species for Europe: Sciurus Meridionalis (Rodentia, Sciuridae). Hystrix 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Menchetti, M.; Russo, D.; Ancillotto, L.; Aloise, G.; Roscioni, F.; Preatoni, D.G.; Loy, A.; Martinoli, A.; Bertolino, S.; et al. Integrating Climate and Land-Use Change Scenarios in Modelling the Future Spread of Invasive Squirrels in Italy. Divers. Distrib. 2019, 25, 644–659. [Google Scholar] [CrossRef] [Green Version]
- Di Febbraro, M.; Martinoli, A.; Russo, D.; Preatoni, D.; Bertolino, S. Modelling the Effects of Climate Change on the Risk of Invasion by Alien Squirrels. Hystrix Ital. J. Mammal. 2016, 27. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-57082-1. [Google Scholar]
- Ministry of the Environment. Threatened Wildlife of Japan—Red Data Book, 2nd ed.; Japan Wildlife Research Center: Tokyo, Japan, 2002. [Google Scholar]
- Mazzamuto, M.V.; Morandini, M.; Panzeri, M.; Wauters, L.A.; Preatoni, D.G.; Martinoli, A. Space Invaders: Effects of Invasive Alien Pallas’s Squirrel on Home Range and Body Mass of Native Red Squirrel. Biol. Invasions 2017, 19, 1863–1877. [Google Scholar] [CrossRef]
- Gurnell, J.; Lurz, P.W.W.; Wauters, A.L. Years of interactions and conflict in Europe: Competition between Eurasian red squirrel. In Red Squirrels: Ecology, Conservation & Management in Europe; Shuttleworth, C.M., Lurz, P.W.W., Hayward, M.W., Eds.; European Squirrel Initiative: Woodbridge, Suffolk, UK, 2015; pp. 19–37. [Google Scholar]
- Messetta, M.L.; Milesi, F.; Guichón, M.L. Impacto de la ardilla de vientre rojo sobre la comunidad de aves en la Región Pampeana, Argentina. Ecol. Austral. 2015, 25, 37–45. [Google Scholar] [CrossRef]
- Zarco, A.; Benitez, V.; Fasola, L.; Funes, G.; Guichón, L. Feeding Habits of the Asiatic Red-Bellied Squirrel Callosciurus erythraeus Introduced in Argentina. Hystrix Ital. J. Mammal. 2018, 29, 223–228. [Google Scholar] [CrossRef]
- Azuma, Y. Nest Predation of the Japanese White-Eye by a Formosan Squirrel. Strix 1998, 16, 175–176. [Google Scholar]
- Setoguchi, M. Food Habits of Red-Bellied Tree Squirrels on a Small Island in Japan. J. Mammal. 1990, 71, 570. [Google Scholar] [CrossRef]
- Bertolino, S.; Mazzoglio, P.J.; Vaiana, M.; Currado, I. Activity Budget and Foraging Behavior of Introduced Callosciurus finlaysonii (Rodentia, Sciuridae) in Italy. J. Mammal. 2004, 85, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Tamura, N.; Ohara, S. Chemical Components of Hardwood Barks Stripped by the Alien Squirrel Callosciurus erythraeus in Japan. J. For. Res. 2005, 10, 429–433. [Google Scholar] [CrossRef]
- Hori, M.; Yamada, M.; Tsunoda, N. Line Census and Gnawing Damage of Introduced Formosan Squirrels (Callosciurus erythraeus taiwanensis) in Urban Forests of Kamakura, Kanagawa, Japan. In Assessment and control of Biological Invasion Risks; Shoukadoh Book Sellers: Kyoto, Japan; IUCN: Gland, Switzerland, 2006; pp. 204–209. [Google Scholar]
- Dong, L.; Ji, M.; Xu, W.; Jiang, Y.; Sun, G.; Ran, J. Relation of Damage and Reproduction of Red-Bellied Squirrels in Plantation. Sichuan J. Zool. 2009, 28. [Google Scholar]
- Chou, F.-S.; Lin, W.-C.; Chen, Y.-H.; Tsai, J.-B. Seed Fate of Castanopsis Indica (Fagaceae) in a Subtropical Evergreen Broadleaved Forest. Bot. Stud. 2011, 52, 6. [Google Scholar]
- Mori, E.; Mazzoglio, P.J.; Rima, P.C.; Aloise, G.; Bertolino, S. Bark-Stripping Damage by Callosciurus finlaysonii Introduced into Italy. Mammalia 2016, 80, 507–514. [Google Scholar] [CrossRef]
- Pedreira, P.A.; Penon, E.; Borgnia, M. Bark Stripping Caused by the Introduced Squirrel Callosciurus erythraeus (Sciuridae) in Argentina. Bosque 2017, 38, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Signorile, A.L.; Evans, J. Damage Caused by the American Grey Squirrel (Sciurus carolinensis) to Agricultural Crops, Poplar Plantations and Semi-Natural Woodland in Piedmont, Italy. For. An. Int. J. For. Res. 2007, 80, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Gao, X.; Jiang, M.; Zhang, Z. Behavioral Adaptation of Pallas’s Squirrels to Germination Schedule and Tannins in Acorns. Behav. Ecol. 2009, 20, 1050–1055. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Xiao, Z.; Guo, C.; Chen, J. Scatter-Hoarding Rodents as Secondary Seed Dispersers of a Frugivore-Dispersed Tree Scleropyrum Wallichianum in a Defaunated Xishuangbanna Tropical Forest, China. Integr. Zool. 2011, 6, 227–234. [Google Scholar] [CrossRef]
- Zhou, Y.; Newman, C.; Xie, Z.; Macdonald, D.W. Peduncles Elicit Large-Mammal Endozoochory in a Dry-Fruited Plant. Ann. Bot. 2013, 112, 85–93. [Google Scholar] [CrossRef]
- Bobadilla, S.Y.; Benitez, V.V.; Guichón, M.L. Asiatic Callosciurus squirrels as Seed Dispersers of Exotic Plants in the Pampas. Curr. Zool. 2016, 62, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Romeo, C.; Wauters, L.A.; Ferrari, N.; Lanfranchi, P.; Martinoli, A.; Pisanu, B.; Preatoni, D.G.; Saino, N. Macroparasite Fauna of Alien Grey Squirrels (Sciurus carolinensis): Composition, Variability and Implications for Native Species. PLoS ONE 2014, 9, e88002. [Google Scholar] [CrossRef] [Green Version]
- Gozzi, A.C.; GuichóN, M.L.; Benitez, V.V.; Lareschi, M. Arthropod Parasites of the Red-Bellied Squirrel Callosciurus erythraeus Introduced into Argentina. Med. Vet. Entomol. 2013, 27, 203–208. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Pisanu, B.; Romeo, C.; Ferrari, N.; Preatoni, D.; Wauters, L.A.; Chapuis, J.-L.; Martinoli, A. Poor Parasite Community of an Invasive Alien Species: Macroparasites of Pallas’s Squirrel in Italy. Ann. Zool. Fenn. 2016, 53, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Tompkins, D.M.; White, A.R.; Boots, M. Ecological Replacement of Native Red Squirrels by Invasive Greys Driven by Disease. Ecol. Lett. 2003, 6, 189–196. [Google Scholar] [CrossRef]
- Sato, H.; Torii, H.; Une, Y.; Ooi, H.-K. A New Rhabditoid Nematode Species in Asian Sciurids, Distinct from Strongyloides robustus in North American Sciurids. J. Parasitol. 2007, 93, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, S.; Miyamoto, A.; Yokohata, Y.; Yasuda, M. Gastrointestinal Parasitic Helminth Fauna of the Pallas’s Squirrel (Callosciurus erythraeus) from the Uto Peninsula, Kumamoto, Kyushu, Japan and Analyses of the Abundance of an Alien Nematode, Strongyloides callosciureus. Jpn. J. Zoo Wildl. Med. 2016, 21, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Matsudate, H.; Miyoshi, Y.; Tamura, N.; Murata, K.; Maruyama, S.; Kimura, J.; Nogami, S.; Maeda, K.; Fukumoto, Y.; Akasako, R.; et al. A survey of the parasitic helminths of alien rodents (belly-banded squirrel Callosciurus erythraeus and nutria Myocastor coypus) in Japan. Jpn. J. Zoo Wildl. Med. 2003, 8, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, M. Perspectives of Host-Parasite Relationships between Rodents and Nematodes in Japan. Mammal. Study 2005, 30, S95–S99. [Google Scholar] [CrossRef]
- Dozières, A.; Pisanu, B.; Gerriet, O.; Lapeyre, C.; Stuyck, J.; Chapuis, J.-L. Macroparasites of Pallas’s Squirrels (Callosciurus erythraeus) Introduced into Europe. Vet. Parasitol. 2010, 172, 172–176. [Google Scholar] [CrossRef]
- Gozzi, A.C.; Guichón, M.L.; Benitez, V.V.; Troyelli, A.; Navone, G.T. Gastro-Intestinal Helminths in the Red-Bellied Squirrel Introduced in Argentina: Accidental Acquisitions and Lack of Specific Parasites. Hystrix Ital. J. Mammal. 2014, 25, 101–106. [Google Scholar]
- Gozzi, A.C.; Lareschi, M.; Navone, G.T.; Guichón, M.L. The Enemy Release Hypothesis and Callosciurus erythraeus in Argentina: Combining Community and Biogeographical Parasitological Studies. Biol. Invasions 2020, 22, 3519–3531. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Shiibashi, T.; Yoshizawa, K.; Murata, K.; Kimura, J.; Maruyama, S.; Hayama, Y.; Yoshida, H.; Nogami, S. Ectoparasites of the Pallas Squirrel, Callosciurus erythraeus, Introduced to Japan. Med. Vet. Entomol. 2004, 18, 61–63. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Yoshizawa, K.; Murata, K.; Shiibashi, T.; Kimura, J.; Maruyama, S.; Hayama, Y.; Yoshida, H.; Nogami, S. The First Record of Sucking Louse, Neohaematopinus Callosciuri, Infesting Pallas Squirrels in Japan. J. Vet. Med. Sci. 2004, 66, 333–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vourc’h, G.; Marmet, J.; Chassagne, M.; Bord, S.; Chapuis, J.-L. Borrelia Burgdorferi Sensu Lato in Siberian Chipmunks (Tamias sibiricus) Introduced in Suburban Forests in France. Vector-Borne Zoonotic Dis. 2007, 7, 637–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Ovidio, D.; Noviello, E.; Pepe, P.; Del Prete, L.; Cringoli, G.; Rinaldi, L. Survey of Hymenolepis spp. in Pet Rodents in Italy. Parasitol. Res. 2015, 114, 4381–4384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passantino, G.; Tursi, M.; Vercelli, C.; Filippi, I.; Decaro, N.; Tinelli, A.; Valente, L.; Leone, R.; Zizzo, N. Systematic Pathologic Findings Report of Callosciurus finlaysonii (Horsfield, 1823) (Rodentia, Sciuridae) Squirrels from Maratea Area (South Italy) to Investigate Species-Specific Pathologies, Reliability of CO2 Euthanasia Method, and Possible Use as Environmental Sentinels. Animals 2020, 10, 1771. [Google Scholar] [CrossRef]
- Durden, L.A.; Musser, G.G. The Mammalian Hosts of the Sucking Lice (Anoplura) of the World: A Host-Parasite List. Bull. Soc. Vector Ecol. 1994, 19, 130–168. [Google Scholar]
- Sato, G.; Kawashima, T.; Kiuchi, M.; Tohya, Y. Novel Cyclovirus Detected in the Intestinal Contents of Taiwan Squirrels (Callosciurus erythraeus thaiwanensis). Virus Genes 2015, 51, 148–151. [Google Scholar] [CrossRef]
- Deng, L.; Li, W.; Yu, X.; Gong, C.; Liu, X.; Zhong, Z.; Xie, N.; Lei, S.; Yu, J.; Fu, H.; et al. First Report of the Human-Pathogenic Enterocytozoon Bieneusi from Red-Bellied Tree Squirrels (Callosciurus erythraeus) in Sichuan, China. PLoS ONE 2016, 11, e0163605. [Google Scholar] [CrossRef]
- Schulze, V.; Lurz, P.W.W.; Ferrari, N.; Romeo, C.; Steele, M.A.; Marino, S.; Mazzamuto, M.V.; Calvignac-Spencer, S.; Schlottau, K.; Beer, M.; et al. Search for Polyoma-, Herpes-, and Bornaviruses in Squirrels of the Family Sciuridae. Virol. J. 2020, 17, 42. [Google Scholar] [CrossRef]
- Hofmannová, L.; Romeo, C.; Štohanzlová, L.; Jirsová, D.; Mazzamuto, M.V.; Wauters, L.A.; Ferrari, N.; Modrý, D. Diversity and Host Specificity of Coccidia (Apicomplexa: Eimeriidae) in Native and Introduced Squirrel Species. Eur. J. Protistol. 2016, 56, 1–14. [Google Scholar] [CrossRef]
- Prediger, J.; Horčičková, M.; Hofmannová, L.; Sak, B.; Ferrari, N.; Mazzamuto, M.V.; Romeo, C.; Wauters, L.A.; McEvoy, J.; Kváč, M. Native and Introduced Squirrels in Italy Host Different Cryptosporidium spp. Eur. J. Protistol. 2017, 61, 64–75. [Google Scholar] [CrossRef]
- Iatta, R.; Immediato, D.; Puttilli, M.R.; Danesi, P.; Passantino, G.; Parisi, A.; Mallia, E.; Otranto, D.; Cafarchia, C. Cryptococcus Neoformans in the Respiratory Tract of Squirrels, Callosciurus Finlaysonii (Rodentia, Sciuridae). Med. Myco. 2015, 53, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Kuo, P.-C. Solving Tree Squirrel Debarking Problems in Taiwan—A Review. In Proceedings of the Tenth Vertebrate Pest. Conference, Monterey, CA, USA, 23–25 February 1982; Marsh, R.E., Ed.; University of California: Davis, CA, USA, 1982. [Google Scholar]
- Pedreira, P.A.; Penon, E.A.; Borgnia, M. Debarking Damage by Alien Pallas’s Squirrel, Callosciurus erythraeus, in Argentina and Its Effects on Tree Growth. South. For. A J. For. Sci. 2020, 82, 118–124. [Google Scholar] [CrossRef]
- Robertson, P.A.; Mill, A.; Novoa, A.; Jeschke, J.M.; Essl, F.; Gallardo, B.; Geist, J.; Jarić, I.; Lambin, X.; Musseau, C.; et al. A Proposed Unified Framework to Describe the Management of Biological Invasions. Biol. Invasions 2020, 22, 2633–2645. [Google Scholar] [CrossRef]
- Wittenberg, R.; Cock, M.J.W. Invasive Alien Species: A Toolkit of Best Prevention and Management Practices; CABI: Wallingford, UK, 2001; ISBN 978-1-84593-332-6. [Google Scholar]
- Wood, D.J.A.; Koprowski, J.L.; Lurz, P.W.W. Tree Squirrel Introduction: A Theoretical Approach with Population Viability Analysis. J. Mammal. 2007, 88, 1271–1279. [Google Scholar] [CrossRef]
- Duboscq-Carra, V.G.; Fernandez, R.D.; Haubrock, P.J.; Dimarco, R.D.; Angulo, E.; Ballesteros-Mejia, L.; Diagne, C.; Courchamp, F.; Nuñez, M.A. Economic Impact of Invasive Alien Species in Argentina: A First National Synthesis. NeoBiota 2021, 67, 329–348. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Panzeri, M.; Wauters, L.A.; Preatoni, D.; Martinoli, A. Knowledge, Management and Optimization: The Use of Live Traps in Control of Non-Native Squirrels. Mammalia 2016, 80, 305–311. [Google Scholar] [CrossRef]
- Bertolino, S.; Ancillotto, L.; Bartolommei, P.; Benassi, G.; Capizzi, D.; Gasperini, S.; Lucchesi, M.; Mori, E.; Scillitani, L.; Sozio, G.; et al. A Framework for Prioritising Present and Potentially Invasive Mammal Species for a National List. NeoBiota 2020, 62, 31–54. [Google Scholar] [CrossRef]
- Chapuis, J.-L.; Dozieres, A.; Pisanu, B.; Gerriet, O.; Berlin, S.; Pauvert, S. Plan. National de Lutte Relatif à L’écureuil à Ventre Rouge (Callosciurus erythraeus) Dans Les Alpes-Maritimes; Muséum National d’Histoire Naturelle: Paris, France, 2011. [Google Scholar]
- Ruesink, J.L.; Parker, I.M.; Groom, M.J.; Kareiva, P.M. Reducing the Risks of Nonindigenous Species Introductions. BioScience 1995, 45, 465–477. [Google Scholar] [CrossRef]
- Epstein, G. Invasive Alien Species Management: A Personal Impasse. Front. Environ. Sci. 2017, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Borgnia, M.; Benitez, V.; Gozzi, C.; Guichón, M.L. La Ardilla de Vientre Rojo En Argentina y El Manejo de Especies Introducidas Como Un Problema Biológico y Social. Ecol. Austral. 2013, 23, 147–155. [Google Scholar] [CrossRef]
- Yasuda, M.; Amano, M. Elimination activity of introduced Pallas’s squirrels Callosciurus erythraeus in the Uto Peninsula, Kumamoto Prefecture, Kyushu Island, Japan. Sciurid. Inf. 2011, 26, 26–27. [Google Scholar]
- Scapin, P.; Ulbano, M.; Ruggiero, C.; Balduzzi, A.; Marsan, A.; Ferrari, N.; Bertolino, S. Surgical Sterilization of Male and Female Grey Squirrels (Sciurus carolinensis) of an Urban Population Introduced in Italy. J. Vet. Med. Sci. 2019, 81, 641–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovesi, P.; Bertolino, S. Human dimension aspects in invasive alien species issues: The case of the failure of the grey squirrel eradication project in Italy. In The Great Reshuffling: Human Dimensions of Invasive Alien Species; McNeely, J.A., Ed.; IUCN Publications Services: Gland, Switzerland; Cambridge, UK, 2001; pp. 113–119. ISBN 978-2-8317-0602-3. [Google Scholar]
- Stuyck, J. Pallas’s squirrel Callosciurus erythraeus (Pallas’ eekhoorn, écureuil de Pallas). In Feasibility of Eradication and Spread Limitation for Species of Union Concern Sensu the EU IAS Regulation (EU 1143/2014) in Belgium. Report Prepared in Support of Implementing the IAS Regulation in Belgium; INBO: Brussel, Belgium, 2019; pp. 40–43. [Google Scholar]


| Country | Year First Introduction | Pathway | Releases | Established | Not Established/Rare | Removed/Eradicated |
|---|---|---|---|---|---|---|
| Callosciurus erythraeus | ||||||
| Japan | 1935 | Zoo | 20 | 13 | 6 | 1 |
| China—Hong Kong | 1960s–1970s | Pet trade | 2 | 2 | ||
| France | 1960s–1974 | Pet trade | 2 | 2 | ||
| Argentina | 1970 | Pet trade | 28 | 23 | 5 | |
| The Netherlands | 1998 | Pet trade | 1 | 1 | ||
| Belgium | <2005 | Zoo/Pet trade | 1 | 1 | ||
| Italy | <2011 | Pet trade | 2 | 1 | 1 | |
| Callosciurus finlaysonii | ||||||
| Japan | 1970 | Pet trade | 1 | 1 | ||
| Italy | 1980s | Pet trade | 2 | 2 | ||
| Singapore | - | - | 1 | 1 | ||
| Species | Country | Parasite/Host Relationship | References |
|---|---|---|---|
| Endoparasites | |||
| Brevistriata callosciuri | Japan | Specific | Matsudate et al. [135]; Miyabe et al. [134] |
| Capillariinae | Italy | Acquired | Mazzamuto et al. [131] |
| Japan | Acquired | Miyabe et al. [134] | |
| Gongylonema neoplasticum | Japan | Specific | Asakawa [136] |
| Hymenolepis sp. | France | Acquired | Doziéres et al. [137] |
| Mastophorus sp. | Belgium | Acquired | Doziéres et al. [137] |
| Pterygodermatites sp. | Argentina | Acquired | Gozzi et al. [138] |
| Rictularia cristata | Japan | Acquired | Miyabe et al. [134] |
| Rodentoxyuris sciuri | Italy | Acquired | Mazzamuto et al. [131] |
| Spiruridae | Italy | Acquired | Mazzamuto et al. [131] |
| Stilestrongylus sp. | Argentina | Acquired | Gozzi et al. [138] |
| Strongyloides callosciureus | Italy | Specific | Mazzamuto et al. [131] |
| Japan | Specific | Sato et al. [133]; Miyabe et al. [134] | |
| Strongyloides sp. | Italy | - | Mazzamuto et al. [131] |
| Japan | - | Matsudate et al. [135] | |
| Trichuris muris | Italy | Acquired | Mazzamuto et al. [131] |
| Ectoparasites | |||
| Androlaelaps fahrenholzi | Argentina | Worldwide | Benitez et al. [65] |
| Ceratophyllus s. sciurorum | Italy | Acquired | Mazzamuto et al. [131] |
| Cheyletus sp. | Argentina | Worldwide | Gozzi et al. [130] |
| Ctenophtalmus agyrtes | Italy | Acquired | Mazzamuto et al. [131] |
| Ctenophtalmus sp. | Italy | Acquired | Mazzamuto et al. [131] |
| Cuterebra sp. | Argentina | Acquired | Gozzi et al. [130] |
| Enderleinellus kumadai | Belgium | Specific | Doziéres et al. [137] |
| France | Specific | Doziéres et al. [137] | |
| Japan | Specific | Durden & Musser [145] | |
| Haemaphysalis flava | Japan | Specific/Acquired | Shinozaki et al. [140] |
| Hoplopleura erismata | Belgium | Specific | Doziéres et al. [137] |
| Ixodes ricinus | Italy | Acquired | Mazzamuto et al. [131] |
| Monopsyllus anisus | Japan | Specific/Acquired | Shinozaki et al. [140] |
| Neohaematopinus callosciuri | Japan | Specific | Shinozaki et al. [141] |
| Nosopsyllus fasciatus | France | Worldwide | Doziéres et al. [137] |
| Ornithonyssus cf. bacoti | Argentina | Worldwide | Gozzi et al. [130] |
| Polygenis rimatus | Argentina | Acquired | Gozzi et al. [130]; Gozzi et al. [139] |
| Trombiculidae | Italy | Acquired | Mazzamuto et al. [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzamuto, M.V.; Wauters, L.A.; Koprowski, J.L. Exotic Pet Trade as a Cause of Biological Invasions: The Case of Tree Squirrels of the Genus Callosciurus. Biology 2021, 10, 1046. https://doi.org/10.3390/biology10101046
Mazzamuto MV, Wauters LA, Koprowski JL. Exotic Pet Trade as a Cause of Biological Invasions: The Case of Tree Squirrels of the Genus Callosciurus. Biology. 2021; 10(10):1046. https://doi.org/10.3390/biology10101046
Chicago/Turabian StyleMazzamuto, Maria Vittoria, Lucas A. Wauters, and John L. Koprowski. 2021. "Exotic Pet Trade as a Cause of Biological Invasions: The Case of Tree Squirrels of the Genus Callosciurus" Biology 10, no. 10: 1046. https://doi.org/10.3390/biology10101046