Abstract
Spinal cord injury (SCI) disrupts the communication between the brain and the spinal circuits that control movement and integrate sensory feedback, which are usually located below the lesion. The disruption of the different anatomical sources of descending motor control and ascending sensory afferents can result in complete or partial, but permanent motor paralysis. For decades, recovery of motor function after long-standing SCI was thought impossible because of the severe and multi-modal failure of these bidirectional communication pathways. This conclusion was supported by overwhelming and disappointing empirical evidence showing poor recovery in people with chronic (>6 months post-injury), severe SCI despite intensive rehabilitation. However, a recent wave of clinical studies has reported unprecedented outcomes in people with both incomplete and complete SCI, independently demonstrating the long-term recovery of voluntary motor function in the chronic stage after SCI. These studies utilized a combination of intensive rehabilitation and electrical spinal cord stimulation (SCS), which was delivered via epidural multi-electrode arrays implanted between the vertebral bone and the dura mater of the lumbosacral spinal cord. SCS has a long history of applications in motor control, which started soon after its first applications as interventional studies in pain management. To date, SCS has been applied in thousands of individuals with neuromotor disorders ranging from multiple sclerosis to SCI. However, even though the motor-enabling effects of SCS were first observed about half a century ago, the lack of a coherent conceptual framework to interpret and expand these clinical findings hindered the evolution of this technology into a clinical therapy. More importantly, it led to substantial variability in the clinical reports ranging from anecdotal to subjective descriptions of motor improvements, without standardized methods and rigorous statistical analyses. For several decades, these limitations clouded the potential of SCS to promote long-term recovery in individuals with SCI. In this chapter, we present the historical background for the development of SCS to treat motor disorders and its evolution toward current applications for neurorehabilitation in individuals with SCI (Sect. 18.1). We then provide an overview of the conjectured mechanisms of action (Sect. 18.2), and how this collective knowledge has been used to develop SCS into a promising approach to treat motor paralysis after SCI, ranging from tonic stimulation to more sophisticated spatiotemporal protocols (Sect. 18.3). Finally, we open up this review to the recent development of non-invasive methods to deliver SCS, namely transcutaneous SCS, and its comparison with epidural SCS in terms of functional effects and underlying mechanisms (Sect. 18.4).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 The Origins of SCS: From Pain to Motor Control
1.1 The Rise of SCS for Chronic Pain Management
SCS was originally developed for the treatment of chronic, intractable pain based on neurophysiological studies suggesting the possibility to inhibit pain fiber input in the spinal cord by stimulation of the larger-diameter sensory fibers [1, 2]. The spinal cord dorsal columns, which contain the longitudinal ascending continuations of cutaneous fibers from many spinal cord segments, appeared as an optimal target for inhibiting pain, in particular because of their easy surgical access from the dorsal aspect of the spinal cord. Subdural electrical stimulation of these structures proved successful in managing pain in cats [3], followed by the first human application of SCS in a patient with cancer [4]. The evolution from subdural to less invasive epidural electrodes, and the development of fully implantable commercial systems led to the FDA approval of epidural SCS for chronic pain management in 1989. Today, SCS accounts for about 70% of all neuromodulation treatments [5].
1.2 First Evidence of Improved Motor Function During SCS: From Multiple Sclerosis to SCI
The ability of SCS to restore function after neuromotor disorders was first unexpectedly observed in 1973 when a subject with motor deficits as a consequence of multiple sclerosis (MS) received SCS therapy to treat intractable pain. Investigators unexpectedly observed that the subject regained volitional control of nearly normal strength of the lower limbs, facilitation of sitting, standing, and ambulation when SCS was active [6]. Four additional implanted MS participants without pain reported a feeling of lightness of the legs during movement, increased endurance during ambulation, as well as the recovery of some voluntary motor function and improved bladder control. Cook improved the technique of electrode placement in the epidural space and implanted more than 200 additional patients with MS within the next few years [7, 8]. Following this first example of SCS-mediated motor improvements, Illis and colleagues introduced the use of SCS for motor disorders to Europe by replicating Cook’s methods. Their first study demonstrated marked improvements in motor, sensory, and bladder function in two participants with MS receiving SCS [9]. The first participant, who had signs of an upper motor neuron lesion and presence of spasticity, regained the ability to walk independently with SCS within the first 24 h of continuous stimulation, while the second participant had marked improvements in sensory function.
Follow-up large-cohort studies by the same group revealed high variability in outcomes that reduced the initial enthusiasm for SCS. Indeed, although motor and sensory improvements surpassed those ever achieved by any other method at the time, it was only 5 out of 19 individuals that experienced these types of improvements [10]. A subsequent study on 90 participants confirmed that these striking improvements in motor function were infrequent and observed only in a few individuals [11]. An objective effect was only seen on bladder dysfunction and limb spasticity. Similarly, studies by other groups showed remarkable effects of SCS on altered motor function after MS, but with variability across subjects. Siegfried and colleagues tested 111 patients with MS with temporary SCS and considered only about 33% of them as responders [12]. Davis and colleagues also reported on 69 MS patients with full implantations [13]. 64% showed improvements in gait, endurance, and muscle strength. Among these patients, nine who were wheelchair users could walk again with SCS.
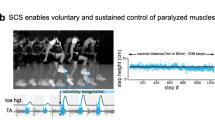
In spite of the variable outcomes of these initial studies, the clinical importance of the potential effects led to a cascade of studies assessing the off-label use of SCS in subjects with a wide variety of motor disorders, including amyotrophic lateral sclerosis, cerebral palsy, traumatic brain injury, dystonia, torticollis, and a few individuals with SCI. Overall, these studies reported improvements in strength, balance, walking, coordination, speech, swallowing, eye movements, and bladder function [12,13,14,15,16]. Initial investigations on SCI focused primarily on the management of spasticity, but also explored secondary effects on autonomic function including bowel control and sexual function, as well as motor capacity [17,18,19,20,21]. More specifically, Waltz and colleagues observed improved motor control in 65% of 303 participants with SCI who received SCS of the cervical region [22]. These improvements in motor control were initially ascribed to a reduction in spasticity enabled by SCS [17]. However, further studies such as the one by Barolat in 1986 revealed that voluntary control of paralyzed muscles was strictly dependent on SCS and stopped immediately when SCS was turned off, independently of changes in spasticity [23] (Fig. 18.1). This effect is discussed in more details later (Sect. 18.3.1.1).
First discovery that SCS enables voluntary movement after SCI in 1986. Pictures of one individual with SCI and with some residual motor control over the left toes and ankle, during an attempt to perform a voluntary left knee extension in the absence (left panel) or presence (right panel) of SCS. The knee angle and EMG activity of quadriceps and hamstrings muscles are represented. During SCS, EMG signals from the hamstrings are affected by stimulation artifacts. Red rectangles indicate the 3 repetitions of the flexion–extension movement, highlighting the EMG activity in the quadriceps, which is facilitated by SCS but voluntarily triggered. Adapted with permission from [23]. All rights reserved
In his observations after treating 1336 individuals with a broad range of disorders over a quarter of a century Waltz reported marked or moderate improvements in a majority of them (Table 18.1) [24]. However, despite the initial unprecedented improvements in motor function observed through SCS, the lack of a conceptual framework kept these observations anecdotal. There was no identified physiological criterion to predict responders to SCS, and a lack of agreement on the optimal electrode implantation site, which ranged from high-cervical (C2) to low-thoracic (T10) vertebral levels. These two factors significantly contributed toward the observed inconsistency in clinical outcomes [12, 13, 25], and led to a declining interest in SCS for motor disorders during the 1990s [26].
1.3 Standardization of SCS Location for Leg Motor Control Following Motor Disorders
A critical contribution to current applications of SCS came from Dimitrijevic and colleagues, who observed that the optimal electrode placement to control leg spasticity was at the T11-L1 vertebral levels, which corresponds to the site of innervation of leg motoneurons in the lumbosacral spinal cord [19]. Indeed, tonic SCS, delivered with higher stimulation amplitudes and lower frequencies than those used for spasticity, could elicit rhythmic, step-like activity in paralyzed muscles of six subjects with chronic, complete SCI [27]. The multi-segmental muscle activity showing consistent rhythmicity and clear alternation between antagonistic muscles was interpreted as the most direct evidence at the time for the existence of central pattern generators (CPGs) in the human spinal cord [27, 28].
These important observations in humans were further supported by investigations using simulations of the biophysical interactions between electrical fields and neural structures, which led to the understanding that SCS recruits primarily proprioceptive afferents [29,30,31]. Proprioceptive afferents then convey mono and poly-synaptic excitatory potentials to spinal motoneurons. To maximize motoneuron activation, SCS electrodes must be therefore placed in positions that favor the direct activation of posterior spinal roots that carry these afferents [32,33,34]. In the case of lower limb movements, these correspond exactly to the optimal location observed by Dimitrijevic. Currently, all scientific investigations that aim at improving lower extremity function via SCS use the same level for epidural electrode placement: below the injury and over the posterior aspect of the lumbar and upper sacral spinal cord, regardless of the level of SCI [34,35,36,37,38,39].
2 Potential Mechanisms of SCS for Motor Control
2.1 General Principles
The generation and modulation of spinal motor activity by SCS results from biophysical phenomena that occur upon the application of electrical fields to the spinal cord, which in turn causes physiological effects in the spinal circuitry. Epidural electrodes are positioned in direct contact with the dura mater. During each stimulation pulse, most of the generated ionic currents flow between the active contacts through the dural sac that contains the spinal cord and roots, owing to the relatively high electrical conductivity of the cerebrospinal fluid [29,30,31, 40,41,42]. Depending on the current flow and the relative localization and orientation of the neural structures within the dural sac, specific subsets of neural structures are depolarized to a level where action potentials are generated according to an all-or-nothing principle. These immediate electrical effects of the stimulation were studied using computer models that are able to solve established physics in arbitrary complex geometrical structures, such as the spinal cord, to calculate the electric current flow and electric potential distribution [29, 31, 40,41,42,43]. This method allows to calculate currents and voltages but does not provide per se an estimation of the response of electrically active neural tissue to an electrical field applied externally. To this end, nerve fiber models utilizing the Hodgkin-Huxley formalism enable to calculate membrane potentials in response to external currents [44]. Such models were used to estimate the sites of maximum depolarization and activation thresholds for individual neurons or their substructures. The electrically activated neurons that lead to motor effects were also investigated by neurophysiological studies in rats and individuals with SCI [28, 29, 38, 45,46,47]. The physiological effects that are caused by electrically activated neurons into their post-synaptic targets involve local and potentially suprasegmental circuits. The underlying mechanisms are not completely clear and still a topic of active research.
2.2 Electrically Activated Neural Structures
The neuronal substructure that is the most excited by external electrical stimulation is the axon. In particular, myelinated and large-diameter axons have the lowest thresholds to stimulation [48]. The axonal depolarization is proportional to the second-order spatial derivative of the electric potential along the axonal path [49, 50]. The stimulation-induced currents within the dural sac primarily flow within the relatively well conductive cerebrospinal fluid, in which the spinal roots are bathed, with maximum current densities in the vicinity of the active electrodes and rapid attenuation when entering the spinal cord [29, 31, 42]. Because of their small size, the lack of myelin sheath, and the fact that the induced current poorly enters into the spinal cord, direct electrical activation of spinal interneurons is highly unlikely to happen with SCS [29]. On the other hand, strong depolarizations are caused in the sensory axons of the longitudinally running lumbar and upper sacral posterior roots of passage that are the closest to the active cathode location [31]. Additional low-threshold sites are created along the sensory axons at the posterior rootlet-spinal cord interface, owing to anatomical inhomogeneities, i.e., electrical conductivity boundaries and changes of the orientation of the axon paths with respect to the electric field [31, 51]. Based on their fiber diameters, electrical activation is likely limited to a subset of posterior root fibers ranging from group Ia muscle spindle afferents to group II afferents, both proprioceptors and cutaneous mechanoreceptors [42].
Upon entering the spinal cord through their posterior rootlets, the sensory axons bifurcate to rostral and caudal projections in the posterior columns. Collaterals connect to locally confined spinal neurons or to relay neurons with ascending axons. There are anatomical differences in the rostral projections between the Ia muscle spindle afferents and the cutaneous afferents that are relevant for their recruitment by SCS [38, 40, 52]. While the rostral projections of the cutaneous afferents can ascend the entire length of the posterior columns, those of the Ia muscle spindle afferents from the lower extremity largely terminate in the upper lumbar and lower thoracic segments to synapse with the relay neurons of Clarke’s column, and in doing so, they occupy deep positions in the white matter [53,54,55]. Therefore, electrical activation of neural structures within the posterior columns—that is limited to its most superficial layers—is essentially restricted to a small population of ascending projections of cutaneous afferents [56]. On the other hand, a large proportion of the total number of the large-diameter proprioceptive afferents can be in theory recruited in the posterior roots or rootlets [31], especially in the lumbosacral and cervical enlargements where the roots cover the entire cord with rootlet projections [31, 40, 42].
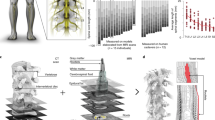
All neurophysiological studies in humans and animals to date support the notion that the motor effects evoked by SCS are triggered by posterior root fiber stimulation. Responses tested by paired pulses applied epidurally with a step-wise decrease of interstimulus intervals clearly demonstrated post-stimulation depression [38, 47, 57], a hallmark of monosynaptic reflexes evoked in proprioceptive afferents [58,59,60]. Complete suppression of responses with repetitive stimulation also rules out the direct electrical activation of motor axons in the anterior roots [38, 40, 49, 61]. Finally, the order of recruitment of lower- and upper extremity muscles with different segmental innervation, either from different segmental cathode positions, with different cathode–anode combinations, or with graded stimulation amplitudes, can be entirely explained by stimulation of the respective posterior roots [34, 38, 40]. Typically, moving the stimulating cathode in the rostral direction away from the segmental posterior root entries increases the response threshold for muscles innervated by motoneuron pools located in these segments, in accordance with earlier predictions by computational models [29,30,31, 40]. For instance, S1-innervated lower leg muscles cannot be recruited by clinically applicable stimulation amplitudes with a cathode located medially over the upper lumbar segments [38]. This can only be explained if the effects of the stimulation are mediated by the posterior roots and not by the longitudinal sensory fiber projections within the posterior columns. Overall, the notion that SCS can be configured to selectively activate proprioceptive afferents of subsets of posterior roots can be used to direct SCS effects toward specific motoneurons to define more targeted stimulation strategies [32,33,34, 39, 62] (Fig. 18.2).
Segmental recruitment of posterior roots and innervated muscles during SCS. a Anatomical representation of the spine, (defined by vertebral body and intervertebral disc heights), lumbosacral spinal cord (aligned with the spine), and segmental innervation probabilities (0%–100%) of lower extremity muscles, reflected by the opacity of their respective colors. These representations correspond to an average over thousands of individuals published in the literature. Adapted with permission from [38]. (Figure published under a Creative Commons license: Fig. 18.2a, licensed under CC-BY 4.0). b Left: mid-sagittal MRI image of the spine and spinal cord of a study participant with SCI, with indications of the vertebral levels, estimated level of the tip of the spinal cord (conus medullaris, yellow line) and electrode array location in this subject (red line). Middle: reconstruction of the location of the electrode array based on MRI and Computed Tomography (CT) scan. Right: Computational model showing the location of the epidural electrode array, epidural fat (yellow), cerebrospinal fluid (dark blue), gray and white matter (gray), and posterior roots (light blue). c Responses of rectus femoris and tibialis anterior to 2‐Hz SCS with incremental amplitudes show the presence of late EMG components in tibialis anterior, but not in rectus femoris, with EES amplitudes greater than two times the response threshold. Adapted with permission from [66]. (Figure published under a Creative Commons license: Fig. 18.2c, licensed under CC-BY 4.0). d Electrophysiological recordings were used to determine optimal electrodes and amplitudes for targeting specific spinal cord regions. EMG responses when delivering single-pulse EES at increasing amplitudes are shown (gray traces). Motor neuron activation maps correspond to optimal amplitudes (black traces). Circular plots report EMG amplitude (in gray scale) at increasing amplitudes (radial axis). White circles show optimal amplitudes; polygons quantify selectivity at this amplitude. c–d: adapted with permission from [34]. All rights reserved
2.3 Evidence for Post-synaptic Activation of Neural Circuits
The directly activated proprioceptive afferents make connections with multiple classes of interneurons and motoneurons in the spinal cord. Therefore, the direct recruitment of proprioceptive afferents will generate synchronized volleys of excitatory post-synaptic potentials into all the neurons directly connected by these fibers. This means that the effects of SCS can occur in multiple neural circuits, having effects that can propagate to both spinal and supraspinal structures. A major goal of current research in SCS is to elucidate the contribution of these activated circuits and their relevance to the effects observed in human clinical trials. In this section, we will discuss only a few of the most studied circuits underlying the effects of SCS, while acknowledging that many more may contribute to the restoration of voluntary motor control.
2.3.1 Recruitment of the Monosynaptic Reflex
In previous paragraphs, we briefly anticipated that most of the motor effects of SCS can be largely explained by assuming the direct recruitment of large proprioceptive afferents. Indeed, Ia-afferents form strong monosynaptic excitatory connections to spinal motoneurons, especially with those innervating extensor muscles. In consequence, each pulse of SCS will generate strong synchronized volleys of excitatory post-synaptic potentials on motoneuron membranes, which can either lead to their direct activation (H-reflex-like responses also known as posterior root-muscle reflexes), or to an increase in their membrane excitability depending on how many afferents are recruited. Since the number of recruited afferents is directly proportional to the strength of the electrical fields, one can argue that stimulation amplitude will then determine whether SCS is in “subthreshold” (i.e., modulating motoneuron membrane excitability) or “supra-threshold” (i.e., directly inducing action potentials in the motoneurons). Therefore, supra-threshold SCS pulses can induce single, distinct H-reflex-like responses in the muscles with a segmental recruitment order. Stimulation of the more rostral roots will mainly induce responses in rostrally innervated muscles, whereas stimulation of the more caudal roots will elicit responses in the more caudally innervated muscles [29, 33, 34, 38, 62]. This property is remarkably robust across animal species and humans as well as the spinal cord region (cervical vs lumbar). This large body of evidence demonstrates that the monosynaptic reflex is certainly involved in the generation of motor outputs during SCS and likely contributes to the facilitation of voluntary motor control by modulating motoneuron excitability.
2.3.2 Recruitment of Excitatory Spinal Circuits
The group I and group II afferent fibers activated by SCS have rich synaptic connections to spinal motoneurons, to first-order interneurons of spinal circuits with a pivotal role in the control of locomotion, as well as to supraspinal circuits—given that residual longitudinal connectivity exists following a trauma [60, 63, 64]. Studies so far are limited to local spinal effects that have been indirectly deduced in individuals with SCI, using trains of stimulation with various frequencies (2–100 Hz) and intensities [57]. As introduced above, the most prominent events are stimulus-triggered, short-latency responses that are predominantly monosynaptic in nature—called posterior root-muscle reflexes—and are most evident at lower stimulation frequencies (i.e., 2–10 Hz) [28, 46, 47, 57, 65]. Low-frequency SCS was also shown to evoke crossed-reflexes in thigh muscle groups [66], posterior root-muscle reflexes with superimposed, delayed-latency electromyographic (EMG) components in flexor muscles (by ~8 ms with respect to the monosynaptic response) [66], and complex long-latency responses (>50 ms) [66, 67] with characteristics reminiscent of the late flexion reflex observed in individuals with chronic SCI [60, 66, 68]. These observations hence suggest that SCS can activate commissural neurons as well as interneurons specific to flexor-related oligo- and poly-synaptic pathways, i.e., interneuron types shown to be essential components of the mammalian lumbar locomotor circuitry [66, 69]. Indeed, previous studies demonstrated that tonic lumbar SCS at frequencies around 30 Hz applied in individuals with chronic, motor complete SCI examined in the supine position could generate periods (10–60 s) of rhythmic EMG activities in the paralyzed lower extremity muscles [27, 47]. The generation of rhythmicity with cycle frequencies compatible with slow to fast walking speeds and various patterns of muscle recruitment, including reciprocity between antagonistic muscles [70] by a sustained stimulation with constant parameters, were interpreted as evidence for the activation of a human CPG for locomotion [27, 71]. It should be noted that the generation of these rhythmic EMG activities required a minimum frequency of 22.5 Hz and rather high SCS amplitudes, about three times the posterior root-muscle reflex threshold of the mid-lumbar-innervated quadriceps muscle group [70]. Such stimulation likely recruits a large proportion of group I and II afferent fibers within the lumbar posterior roots. The antidromic action potentials carried along these afferents toward the periphery likely cancel a large part of the naturally generated, orthodromically traveling action potentials from proprioceptors [71, 72]. The generation of rhythmic activity independent from phasic peripheral feedback (largely canceled following antidromic collision) is essential in the demonstration of centrally generated rhythmicity and significant from a neuroscientific point of view. However, the blocking of proprioceptive feedback required for generating adaptive movements limits the applicability of such high stimulation intensities in neurorehabilitation approaches [72, 73].
2.3.3 Recruitment of Inhibitory Spinal Circuits
Despite their historical use in spinal spasticity [6, 21, 74], the recruitment of inhibitory spinal circuits by SCS has received less attention. A phenomenon observed with low SCS frequencies sheds some light on the activation of inhibitory interneurons [49]. Stimulation at around 16 Hz can produce specific modulation patterns of repetitively evoked posterior root-muscle reflexes, with every other response being attenuated, resulting in simple periodic patterns. The rhythmic attenuation of responses without changes in stimulation amplitude and the reciprocal organization of these patterns between antagonistic muscles suggests the recruitment of inhibitory circuits involving Renshaw cells and Ia inhibitory interneurons [49]. Indeed, similarly to monosynaptic connections to the motoneurons, Ia-inhibitory interneurons are known to have direct inputs from Ia-afferents [75, 76]. These interneurons inhibit the motoneurons that innervate antagonistic muscles. Therefore, the potentiation of these inhibitory cells, or even their direct post-synaptic activation by SCS, is likely involved in the alternation of agonist and antagonist muscle activations, which can, in turn, contribute to the production and modulation of coordinated movements.
2.4 Lessons from Animal Studies
2.4.1 Recruitment of Different Reflex Circuits by SCS in Animal Studies
In intact and spinalized rats, single-pulse SCS of the lumbosacral spinal cord evoked composite EMG responses in the hindlimb muscles with a succession of stimulus-triggered, partially overlapping potentials [29, 45, 77]. Three physiologically different components were suggested, and distinguished according to the relative latencies of their major EMG peaks: an early, middle, and late-latency response. The early response is a direct, M-wave-like response [60], elicited by direct electrical stimulation of motor axons within the ventral roots—a response type not evoked in humans with electrodes placed over the midline (see above). The middle response is well documented to be a monosynaptic reflex, likely corresponding to the monosynaptic posterior root-muscle reflex in humans [47]. The late response occurs with an additional delay of 4–5 ms with respect to the middle response, distinguishing it as an oligosynaptic spinal reflex [38]. There is the uncertainty of the origin of the late response, but it was suggested to involve group II muscle afferents [29] or the flexor reflex afferents [45, 77].
2.4.2 Recruitment of Locomotor Circuits by SCS in Animal Studies
Early animal studies employing spinal cord and/or root stimulation were conducted in cats with the aim to investigate the intrinsic capability of the lumbar spinal cord to generate the rhythm and pattern underlying hindlimb locomotion. Stimulation of bilateral pairs of lumbar dorsal roots, typically with 30–50 Hz, induced rhythmic alternating activity in spinal preparations after elimination of phasic inputs from the hindlimbs (through deafferentation or curarization), and hence demonstrated the existence of a CPG [78]. Similar patterns were observed in acutely spinalized cats pretreated with L-DOPA and in chronic spinal cats acutely decerebrated but without administration of drugs. Another classical study demonstrated that subdural and epidural stimulation over the posterior aspect of the lumbar spinal cord could elicit stepping in acutely spinalized cats suspended over a moving treadmill without pharmacological manipulation [79]. Electrodes positioned over the spinal cord midline or laterally over the dorsal root entry zones were both effective. This animal study was perhaps one of the first to suggest the value of SCS for locomotor rehabilitation, as it could be suitable for “a degree of “exercise” and perhaps provide sufficient force to propel the subject as long as postural support is provided” [79]. Later studies in rodents and non-human primates provided further ground for the development of SCS into a tool for enabling locomotion after SCI (for a detailed review in rodent models, see part I, Chap. 4: “Multisystem Neurorehabilitation in Rodents with Spinal Cord Injury”).
3 The Evolution of SCS into a Neuroprosthetic Technology and a Neurorehabilitation Therapy
In the previous sections, we discussed the early history of human applications of SCS in motor disorders, how empirical observations highlighted the potential of SCS to enable voluntary motor control, and its potential mechanisms of action. In this section, we bring all this information together to discuss how the concept of SCS shifted from a neurophysiological tool to activate CPGs to a therapy capable of amplifying residual voluntary motor control [80]. Currently, the application of SCS from the epidural space located over the posterior aspect of the lumbosacral spinal cord represents a state-of-the-art neuromodulation technique for facilitating lower limb motor control after SCI. Here, we report the technological innovations that enabled this transition toward a neuroprosthetic solution for motor impairments and a neurorehabilitation therapy for long-term recovery. In particular, we discuss different approaches for delivering SCS, from tonic stimulation to more sophisticated spatiotemporal stimulation protocols, which can be either applied at a pre-defined pace, triggered by external events, or adjusted in closed loop.
3.1 Tonic SCS for the Recovery of Voluntary Motor Control in People with SCI
3.1.1 The Initial Discovery that SCS Enables Voluntary Motor Control
The very first discovery that SCS may be used as a motor-enabling neuromodulation tool after SCI is attributed to Barolat and colleagues in 1986 [23]. This case study showed that one subject with incomplete SCI regained voluntary motor control in the presence of SCS after several months of stimulation (Fig. 18.1). Specifically, SCS allowed the subject to perform a full knee extension against gravity, despite the complete absence of voluntary activity in the thigh muscles in the absence of SCS. This effect was present only when the stimulation was turned on, illustrating for the first time an immediate effect of SCS for enabling motor function after SCI. Importantly, this first observation of improved motor control did not involve the triggering of automatic stepping patterns, which was the main focus of animal research in the field. Instead, SCS-enabled single-joint voluntary movements, which is still the main clinical outcome of modern clinical trials.
3.1.2 SCS as a Tool to Trigger Movement Primitives
Several years later, Dimitrijevic and colleagues demonstrated in six subjects with complete SCI that SCS delivered over the lumbar spinal cord (T11-L1 vertebrae) in supine position produced rhythmic EMG responses and flexion–extension leg movements resembling stepping [27]. Stimulation was applied at relatively high amplitudes (5–9 V) and for frequencies between 25 and 60 Hz. Beyond its potential prosthetic implications, this discovery provided indirect evidence for the existence of a CPG in humans, defined as a circuit within the spinal cord capable of generating rhythmic outputs in response to a tonic input (i.e., a continuous pulse train at a given frequency without rhythmic modulations).
In addition to the rhythmic movements induced by stimulation of the CPG, other simpler movements could be produced. For example, Jilge and colleagues showed in five subjects that tonic SCS at frequencies between 5 and 15 Hz induced bilateral extension of the lower limbs [81]. Minassian and colleagues next demonstrated in ten subjects that tonic SCS at different frequencies can switch the functional state of spinal circuits between distinct functional units that produce muscle synergies associated with either bilateral extension of the lower limbs (for frequencies of 5–15 Hz) or rhythmic stepping-like movements (for frequencies of 25–50 Hz) [47]. This latter study was the first one to coin the term “neuroprostheses” in the context of SCS: “This study opens the possibility for developing neuroprostheses for activation of inherent spinal networks involved in generating functional synergistic movements using a single electrode implanted in a localized and stable region”.
However, it is important to highlight that the recruitment of CPGs or other movement primitives should not be taken as a goal to produce automatic, non-voluntary stepping (which may not be relevant as a clinical outcome), but rather as a demonstration that SCS can engage the spinal circuitry that is necessary to produce complex motor patterns such as locomotion. For this reason, subsequent studies investigated the combination of SCS with physical training as a means to improve the capacity of residual descending inputs to regain control over spared spinal circuits.
3.1.3 The Combination of SCS with Training and the Re-Discovery that SCS Enables Voluntary Motor Control
The first combination of SCS with partial weight-bearing locomotor training was performed by Herman and colleagues in a subject with incomplete SCI [82, 83], and later expanded to a second subject [84]. Both participants had incomplete SCI with no independent ambulatory function and were graded as AIS C with low lower extremity motor scores—following the American Spinal Injury Association (ASIA) Impairment Scale (AIS). Treadmill stepping alone improved gait performance on the treadmill but did not improve overground walking capabilities. Instead, the addition of SCS led to the immediate facilitation of walking, as well as further training-related gait improvements and a reduced sense of effort. Despite remarkable improvements in walking capability, there was no change in muscle strength or lower extremity motor scores when the stimulation was turned off, suggesting that this approach was insufficient to trigger neuroplasticity mechanisms in the tested subjects.
The next milestone in the application of SCS to neurorehabilitation came from a case study by Harkema and colleagues in 2011, which aimed at promoting standing and assisted treadmill stepping in a subject with motor-complete, sensory-incomplete SCI (AIS-B) [85]. SCS-enhanced rhythmic EMG activity during assisted treadmill stepping, evoked sustained activation patterns in lower extremity muscles, and allowed independent, full weight-bearing standing after 80 sessions of intensive training. An additional major outcome of the study was the incidental re-discovery of the so-called motor-enabling effect of SCS, initially observed by Barolat in 1986. Indeed, the participant reported that SCS enabled him to perform voluntary movements of paralyzed muscles, including toe extension, ankle dorsiflexion, and leg flexion. As in 1986, this effect was present only when the stimulation was on.
The investigators focused on this motor-enabling effect in a subsequent study involving intensive training of voluntary leg movements under SCS in four participants with motor complete SCI (two graded AIS-A, and two AIS-B), including the participant from the original study [35]. All new participants were able to voluntarily induce movement when SCS was applied from the very first day and without any training, even for the two sensory and motor complete subjects (Fig. 18.3).
Tonic SCS immediately enables voluntary motor control. a Lower extremity EMG activity during voluntary movement attempts (ankle dorsiflexion) without and with SCS in four individuals with clinically determined motor complete SCI. Electrode representations show cathodes (gray) and anodes (black). Stimulation frequency: 25–30 Hz. Muscles, surface EMG: intercostal sixth rib (IC), tibialis anterior (TA), soleus (SOL); fine wire EMG: iliopsoas (IL), extensor digitorum longus (EDL), extensor hallucis longus (EHL). Gray highlighted: active ‘flexion/extension’ period. b Left leg force and iliopsoas, vastus lateralis and intercostals EMG activity generated during a low (20%), medium (60%), and high (100%) effort of hip/knee flexion with SCS from patient 3. Gray shading: force duration. c Volitional modulation in EMG activity by an individual with motor complete SCI during manually assisted stepping (40% body weight support, 1.07 m/s) in the presence of SCS. Initial steps show EMG pattern while the subject (patient 3) is not thinking about stepping. Section within the red dashed lines show the period of steps while the subject is consciously thinking about stepping and facilitating each step (with voluntary intent). Adapted with permission from [35]. All rights reserved
With training enhanced by SCS, all participants improved over time and became able to control movements based on visual or auditory cues. Three of them could generate graded levels of force in at least one leg, and two could modulate EMG activity during assisted treadmill stepping and SCS by thinking about moving the legs. Additionally, all participants became able to perform full weight-bearing standing with SCS after 80 sessions of stand training [86]. A follow-up study found that rehabilitation in the presence of SCS needs to be task-specific, and that stand and step training leads to different functional outcomes [87]. After completion of the study, one of the participants (AIS-B) was enrolled to receive additional activity-based training with SCS, and his voluntary leg motor control progressively improved throughout the 3.7 years of training, to a level such that he could produce voluntary leg movement and standing even with SCS turned off [88].
With the goal to confirm the motor-enabling effect of SCS on participants with severe SCI, an independent group at the Mayo Clinic conducted a 2-week, 8-session study on a participant with chronic, complete SCI (AIS-A) attempting volitional control of leg movements with SCS [89]. Stimulation enabled voluntary knee flexion, initiation and termination of rhythmic leg movements, full weight-bearing standing, and voluntary generation of step-like movements while stationary in an upright position with body-weight support. These abilities were present only when the stimulation was turned on.
3.2 Spatiotemporal SCS for Neuroprosthetics and Neurorehabilitation in Animal Models of SCI
In parallel with these investigations utilizing tonic SCS in humans, several studies in animal models of SCI were laying the groundwork for a new stimulation paradigm that would combine the ability to promote voluntary motor control and overt synergistic movements during functional tasks: spatiotemporal SCS. So far, we described applications in which SCS was applied tonically, i.e., stimulation parameters such as amplitude, frequency, pulse width, and electrode configurations (choice of anodes and cathodes) were set manually by the experimenter at the beginning of a trial and were kept constant across consecutive steps. In real-life situations, however, the fine control of gait requires the ability to modulate muscle activity and kinematic outputs depending on the environment, task requirements, or levels of fatigue, which motivated the development of spatiotemporal SCS.
3.2.1 Spatiotemporal SCS Controlled by Residual Kinematics
During SCS-enabled locomotion, this adjustment can be done artificially by linking the task requirements and the observed kinematics with the stimulation parameters used to control SCS in real time. In a first pioneering study, Wenger and colleagues established the first proof-of-concept closed-loop SCS in rat models of SCI [90]. They identified a linear relationship between SCS frequency and the elicited step height, which they exploited as part of a closed-loop proportional-integral (PI) controller. This controller adjusted SCS frequency in real-time and for each step based on the desired and observed step heights, which depended on the task requirements and environment. For example, climbing a staircase required a higher step height than overground walking on flat surfaces. The observed kinematics were obtained by means of a motion capture system that monitored the 3D position of infrared-reflective markers placed on the joints of the hindlimb. This technological solution shows the possibility to develop intelligent systems that can support an individual in modulating motor output by adjusting SCS parameters in real time.
In a subsequent study, the same group introduced a new concept of real-time control of SCS parameters, which selected spatially-distinct sets of electrodes during different phases of the gait cycle. This protocol aimed at independently activating specific muscle synergies such as leg flexion or extension at the appropriate time, a concept termed spatiotemporal SCS [62]. Specifically, they aimed at replicating the dynamics of motoneuron activation underlying gait in non-injured animals, which was indirectly inferred from the EMG activity of several key hindlimb muscles innervated at various segments within the lumbosacral spinal cord. The obtained spatiotemporal maps of motoneuron activation during bipedal locomotion highlighted two spatially distinct activation patterns associated with major muscle synergies, and with, respectively, flexion and extension movements of the hindlimb, hereafter referred to as “hotspots”.
To reactivate these hotspots in animals with SCI, spatially specific electrode configurations were first selected to recruit the dorsal roots projecting to the corresponding spinal segments. Next, kinematic gait events corresponding to the time when the animal places the foot on the ground (“foot strike”) and lifts it off (“foot off”) were extracted in real-time using the motion capture system described previously. Extracting these gait events was possible thanks to the residual kinematics of the animals with incomplete SCI placed in a body-weight support system. In this new paradigm, SCS was not applied tonically to the lumbosacral spinal cord, but as short pulse trains (lasting a few hundreds of milliseconds) triggered by these extracted gait events. Specifically, electrode configurations able to activate the upper lumbar and sacral segments of the spinal cord (associated with hindlimb flexion and extension, respectively) were triggered by the “foot off” and “foot strike” gait events, respectively. In addition, SCS was delivered at an amplitude sufficiently high to elicit motor outputs through the generation of powerful spinal reflexes, which supported the animals in movement execution.
This study demonstrated that spatiotemporal SCS allows the facilitation of specific movement phases through targeted activation of the appropriate motoneuron pools through spinal circuits during gait, which can be achieved using epidural multi-electrode arrays. This approach also enables to reduce muscle co-activations observed during tonic SCS, to use higher stimulation amplitudes that can provide better weight-bearing capacity, and to vary stimulation frequency throughout the gait cycle depending on the desired functional outcomes.
3.2.2 Spatiotemporal SCS Controlled by Brain Signals
The principles underlying spatiotemporal SCS were then tested in non-human primates, which represent the most suitable animal model for the translation to humans because of the unique organization of the corticospinal tract in these species [91, 92]. In their study, Capogrosso and colleagues extracted motor signals from intracortical recordings in macaque monkeys with SCI to control spatiotemporal SCS, thereby pioneering the concept of a “brain-spine interface” [33]. Specifically, a 96-channel intracortical microelectrode array (Utah array) was surgically implanted into the hindlimb area of the primary motor cortex, which sends motor commands down to the spinal cord. Thanks to a state-of-the-art wireless neuronal amplifier (able to amplify and broadcast wirelessly 96 channels of neuronal data at a sampling rate of 20 kHz), they recorded the spiking activity across the 96 electrodes while animals performed treadmill and overground locomotion. This neuronal activity was used as an input to a machine learning algorithm (a discrete classifier) able to identify neural states associated with flexion or extension of the hindlimb. At the spinal cord level, the animals were implanted with an epidural multi-electrode array designed specifically to cover the lumbosacral segments of the macaque spinal cord. The spinal electrode array was in turn connected to a modified version of an implantable pulse generator (IPG) clinically approved for deep brain stimulation. Modifications to the IPG firmware provided real-time control over stimulation parameters such as electrode configurations, amplitude, and frequency.
This technological framework enabled the implementation of brain-triggered spatiotemporal SCS in freely moving non-human primates. The same principles as used in rats to optimize SCS were extended to non-human primates [32], and stimulation protocols facilitating flexion or extension of the leg were extracted. After a first proof-of-concept in intact animals, two macaque monkeys received a unilateral corticospinal tract lesion that left one hindlimb completely paralyzed. As early as a few days after the experimental lesion, the brain-spine interface enabled to reestablish both treadmill and overground locomotion, with the paralyzed hindlimb moving in coordination with the three other limbs.
This study brought important advancements, both technologically and scientifically. On the technological side, it demonstrated that it is possible to implement brain-triggered spatiotemporal SCS with currently available technologies that are ready for human use. From a scientific standpoint, it demonstrated that spatiotemporal SCS immediately restored weight-bearing locomotion as early as six days post-injury in non-human primates, which bears substantial clinical relevance. The concept of brain-controlled SCS for rehabilitation was further developed by Bonizzato and colleagues who linked cortical ensemble activity to the amplitude of SCS in rat models of SCI and showed a more pronounced and faster recovery of locomotion when training with brain-controlled SCS compared to tonic SCS [93].
In summary, this series of studies laid the technological and scientific premises for the application of spatiotemporal SCS to humans with SCI. First, spatiotemporal SCS provides a way to activate different spinal cord locations and thus different muscle synergies at different phases during the gait cycle or any other motor task. Next, these different stimulation protocols can be triggered based on residual kinematics or neuronal signals to enable a smooth integration into the ongoing locomotor activity. Finally, closed-loop control policies can be additionally used to adjust various parameters such as stimulation amplitude or frequency in real-time to adapt to task requirements and environmental constraints.
3.3 Tonic and Spatiotemporal SCS Combined with Intensive Rehabilitation Restore Independent Overground Walking in People with SCI
The year 2018 marked a milestone for the application of SCS in people with SCI. For the first time, three groups in parallel demonstrated in a total of six subjects with chronic, severe SCI that SCS, delivered with either tonic or spatiotemporal protocols and combined with intensive rehabilitation, could enable independent overground walking [34, 37, 94].
3.3.1 Tonic SCS
At the Kentucky Spinal Cord Injury Center and the University of Louisville, Angeli and colleagues enrolled four participants with motor-complete SCI (two AIS-A, two AIS-B) who performed training sessions for standing, treadmill stepping with body-weight support and manual assistance, and overground walking when possible, all in the presence of tonic SCS [94]. All four participants achieved assisted standing and improved trunk stability in the sitting position in the presence of SCS and after several weeks of training. Most importantly, the two participants with motor-complete, sensory-incomplete SCI (AIS-B) achieved the ability to walk overground with tonic SCS after 278 and 81 training sessions respectively, over a period of 85 and 15 weeks. Walking only occurred when SCS was turned on, and while the participant consciously intended to walk. After 147 sessions, the second participant was able to walk independently with a walker and with SCS, which was an unprecedented level of recovery for a person with motor-complete SCI.
In parallel, at the Mayo Clinic, Gill and colleagues enrolled an individual with chronic motor- and sensory-complete SCI (AIS-A), who had previously trained to perform step-like movements with SCS in a side-lying position [89] to receive additional motor task training with tonic SCS [37]. After 43 weeks of training and in the presence of tonic SCS, this participant was able to stand, step on a treadmill without body-weight support, and walk overground with a walker and assistance of a physiotherapist for hip stability, for the first time in a participant graded AIS-A in the chronic state of SCI. In a recent follow-up study, Gill and colleagues also showed that maximizing participants’ intention to walk and minimizing body-weight support during training with tonic SCS improved independence and decreased the need for external assistance by a physiotherapist (Gill et al. 2020). Conversely to the earlier study by Rejc and colleagues [87], dynamic training combining the repetition of different motor tasks found positive effects on both stand and gait performance simultaneously.
Clinical studies by these two groups illustrated the potential of tonic SCS combined with several months of intensive rehabilitation for restoring motor function after SCI.
3.3.2 Spatiotemporal SCS
Meanwhile, at the Lausanne University Hospital and Ecole Polytechnique Fédérale de Lausanne (EPFL) in Switzerland, Wagner and colleagues pioneered the use of spatiotemporal SCS in three participants with chronic, incomplete but severe SCI (one AIS-D and two AIS-C, including one with motor scores of 0 in all key leg muscles but with remaining sphincter control) [34] (Fig. 18.4). They demonstrated both an immediate facilitation of body-weight-supported walking and long-term recovery of motor function even in the absence of SCS. This strategy leveraged the IPG with real-time control capabilities previously tested in non-human primates [33], which was connected to the same 16-electrode array as used in the other clinical studies cited above (Specify 5-6-5, Medtronic, clinically approved for the treatment of chronic pain). Taking inspiration from their previous methodology in rodents and non-human primates [32], they developed a stimulation protocol that alternated between the swing, weight acceptance, and propulsion phases of the right and left legs at appropriate times and amplitudes during the gait cycle. Each functionality was associated with a stimulation pattern consisting of a spatially specific set of anodes and cathodes optimized to recruit the associated posterior roots, and with a stimulation amplitude and frequency that further maximized the activation of the desired muscle synergy. For example, stimulation frequencies between 40 and 120 Hz tended to better promote a whole-leg flexion synergy (at the hip, knee, and ankle joints simultaneously), while frequencies of 20–30 Hz preferentially recruited functional knee, and ankle extensors. Finally, the alternation of stimulation patterns could either be delivered automatically at a pre-defined sequence and pace, or they could be triggered in real time by residual kinematics for people with sufficient residual control. Movement feedback used to trigger SCS was initially obtained from an infrared-based 3D motion capture system as previously shown in rodents [62], and later by wearable sensors containing inertial measurement units (IMU) placed on the subjects’ feet. Such sensors, along with appropriate algorithms, enabled to extract the foot inclination angle as the participant attempted to initiate movement and to trigger a stimulation pattern that enabled flexion of the corresponding leg.
Spatiotemporal SCS immediately enables independent walking. a Top: the three muscle synergies underlying human walking, and which can be targeted by SCS. Bottom: typical sequence of spatiotemporal SCS and associated parameters for immediate facilitation of walking after SCI. b Chrono-photography, tibialis anterior EMG activity and foot vertical position during overground walking with body-weight support and walking sticks while SCS is switched on, then off, then on in a subject with severe SCI. c Overground walking when a subject with incomplete SCI but completely paralyzed left leg is asked to perform first normal and then exaggerated step heights. d Consecutive values of step height and EMG activity over 60 min of walking with EES (1 km). BWS: body-weight support. Adapted with permission from [34]. All rights reserved
This spatiotemporal SCS paradigm, combined with a cable-based robotic body-weight support system, allowed a wide variety of locomotor tasks both on a treadmill and overground in the three participants with chronic SCI at the cervical level. One participant (AIS-C) had complete motor paralysis on the left leg but residual activity on the right, the second (AIS-D) had paralysis in the leg flexor muscles, and the third (AIS-C, based on the presence of sphincter contraction) had motor-complete paralysis in both legs. Spatiotemporal SCS immediately (i.e., without training) facilitated EMG activity underlying locomotion in otherwise inactive or poorly active leg muscles and enabled participants to walk overground with assistive devices and body-weight support. Participants could voluntarily modulate the effect of the stimulation by exaggerating step elevations, could walk at different speeds and could cover distances of up to 1.2 km on a treadmill without deterioration of kinematics or muscle activity. The first two participants regained the ability to transition from sitting to standing and to walk independently with crutches without SCS or body-weight support. Neurological recovery, tested according to clinical standards and without SCS, was observed to different degrees in all three participants. The first participant improved from AIS-C to AIS-D and gained 16 points in his lower extremity motor scores (from 14 to 30, maximum of 50). The second participant gained 11 points (from 25 to 36). The third participant gained four points (0–4). Although he was not able to perform voluntary movements against gravity in the absence of SCS, the researchers observed an increase in the maximum isometric torques that the participant was able to produce in the presence of SCS. Although the investigators did not attempt to train participants with tonic SCS, they performed a comparison of the immediate facilitation of locomotor activity with tonic versus spatiotemporal SCS. In the three reported participants, tonic SCS created an important co-activation of antagonistic muscles preventing smooth locomotion. Additionally, it disrupted the residual proprioceptive inputs to the spinal cord and the brain, thereby blocking important feedback cues to the spinal locomotor circuitry as well as impairing the conscious perception of the lower limbs in space [72].
In a recent study by the same group, Rowald and colleagues expanded their approach to people with motor-complete SCI (two AIS-A, one AIS-B), who were implanted with a new 16-electrode array specifically designed for the rehabilitation of both leg and trunk motor function after severe SCI [39]. Their approach also involved the development of personalized computational models of the spinal cord derived from structural and functional magnetic resonance imaging (MRI). This study demonstrated that spatiotemporal SCS immediately enables (i.e., within a week of using SCS) powerful facilitation of walking even in motor-complete participants, whereas similar functional outcomes could only be achieved after several months of intensive training using tonic SCS [37, 94]. Furthermore, it provides a path forward in the refinement of neurotechnologies for delivering SCS, ranging from new electrodes arrays and personalized computational models of the spinal cord to versatile software platforms for configuration and use of spatiotemporal SCS by non-experts. The future deployment of such technologies in widespread clinical practice will require the additional development of new implantable neurostimulators and automated pipelines for optimizing SCS parameters.
3.3.3 Limitations of Locomotor Rehabilitation Facilitated by SCS
Improvements in motor function mediated by SCS require high-intensity neurorehabilitation sessions, spread over a time period that is much longer than provided in current clinical practice and covered by insurance. For SCS to become a clinically accepted method for augmenting rehabilitation outcomes, the duration of the rehabilitation phase should be therefore considerably shorter. This could be achieved for example by starting neuromodulation therapies in the sub-acute phase after the injury, which would leverage the intrinsic capacity of the spinal cord to reorganize. In individuals living with a chronic SCI, combining SCS with pharmacological interventions will likely further improve and accelerate rehabilitation outcomes [95, 96]. Administration of pharmacological agents would thereby mimic the effects of neurotransmitters such as serotonin and dopamine, which are essential for locomotion. These neurotransmitters are synthesized in the brainstem and the posterior hypothalamus, but their descending axons become partially separated from the lumbar spinal cord after SCI. Finally, all subjects with severe SCI who achieved overground walking with SCS required their arms to maintain balance using either a walker or crutches. This means that their arms cannot easily serve other purposes, such as reaching for an object and carrying it from one spot to another, potentially limiting the usability of this technology in certain daily life situations. To improve dynamic balance, SCS protocols will need to target additional muscle groups involved in hip/trunk movement and stabilization, such as leg abductors and adductors, as well as the quadratus lumborum and the paraspinal muscles. In fact, there is early demonstration that multi-electrode arrays placed over the low-thoracic and lumbosacral spinal segments, combined with activity-specific stimulation programs, can augment the control of both trunk and leg movements in individuals with chronic, motor complete spinal cord injury [39].
3.4 Other Recent Studies of SCS for Improving Motor and Autonomic Functions After SCI
Following the three seminal studies from 2018, which focused on overground walking, several groups sought to improve a wider range of motor functions and additionally target autonomic functions.
In terms of motor functions, trunk stability turned out to be a key element to improve in motor- and sensory-complete SCI, as already shown by Angeli and colleagues in 2018 [94]. Later, Gill and colleagues also demonstrated in two participants with motor- and sensory-complete SCI that SCS could improve seated reaching distance [97]. On the technological side, Rowald and colleagues showed that a longer electrode array could target both trunk and lower limb motor functions in subjects with complete SCI and considerably improve several daily living and leisure activities that critically require trunk stability [39]. At the University of Minnesota, Darrow and colleagues showed in two female participants with chronic motor- and sensory-complete SCI (AIS-A) that tonic SCS could immediately enable volitional leg movements [98]. In a follow-up study, Pena Pino and colleagues studied the effect of long-term exposure to tonic SCS without intensive neurorehabilitation [99]. After one month of optimization of various stimulation programs for volitional motor control, spasticity, and autonomic functions, participants were allowed to use SCS at home as much as 24 h a day during their daily living activities. Out of seven participants with motor-complete SCI, four of them (all graded AIS-A) recovered the ability to perform voluntary movements even without SCS after a period ranging from 3 to 13 months. Importantly, these movements were not present at every clinical visit, showing variability over time in these motor effects. Even more importantly, higher levels of spasticity seemed to correlate positively with the recovery of voluntary movements. These results add up to the recovery without SCS observed by Rejc and colleagues [88], and independently by Wagner and colleagues [34].
In terms of autonomic functions, Darrow and colleagues showed that SCS improved bowel-bladder synergy in their two participants, with SCS, and cardiovascular function in one of the two participants who had otherwise drops in blood pressure during tilt-table tests [98]. This same participant also reported the ability to achieve orgasm during sexual intercourse when SCS was on or immediately after it was turned off. Although a thorough review is beyond the scope of this chapter, we would like to highlight that SCS has been shown to improve bladder function [21, 98, 100], body composition, and metabolism [101], and blood pressure [98, 102,103,104]. Targeting such autonomic functions is of tremendous importance for improving the quality of life of people with SCI.
3.5 Comparison Between SCS and Functional Electrical Stimulation (FES)
Functional Electrical Stimulation is an established technology that targets efferent axons innervating specific muscles to produce a desired movement, using electrical stimulation applied at the surface of the skin [105,106,107] or with leads implanted in the periphery [108, 109]. FES has important clinical applications in hemiplegia, used for example as a commercially available foot-drop stimulator, and for the rehabilitation of upper extremity motor function. Moreover, it has been extensively used in research applications for SCI [107, 110,111,112], but did not become a standard clinical practice for this condition. In this paragraph, we discuss the conceptual and practical differences between SCS and FES.
3.5.1 Conceptual Differences: Stimulation of Muscles Versus Spinal Circuits
Functional Electrical Stimulation aims at generating force and movement by recruiting the efferent axons that innervate muscle fibers via pulses of electrical stimulation. This direct recruitment of muscles enables a high degree of controllability because each targeted muscle can be independently stimulated. However, the stimulation patterns required to coordinate a functional movement can be extremely complex and are gravity-dependent [110]. Therefore, a specific set of parameters can only work for a pre-determined movement but can hardly be generalized [108]. This aspect significantly increases the complexity of FES systems, as they must be specifically tuned for each task.
Conceptually, SCS works very differently than FES, as SCS engages surviving spinal circuits below the lesion via their input fibers, the excitatory sensory afferents. SCS, therefore, overcomes some of the limitations of FES because it requires simple stimulation protocols that leverage existing neural architectures to perform complex movements of a whole limb [33, 34, 113]. Indeed, excitatory spinal circuits producing synergistic movements receive rich innervation from the primary afferents stimulated by SCS [62, 114] and a single Ia afferents connects to all the motoneurons of the homonymous muscle and up to 60% of synergistic motoneurons even at different joints [115].
Another key difference is that FES imposes a specific movement according to a preprogrammed pattern, irrespective of the subject’s voluntary intention. On the other hand, SCS protocols are thought to synergistically act and enhance residual voluntary inputs. Motor outputs can then be modulated and naturally shaped by movement-specific feedback [75] as well as volitional contributions [34, 35].
Concerning the ability to produce large forces, FES suffers from the “inverse recruitment effect”. Since large axons have a lower threshold to electrical stimulation than smaller diameter fibers [48], FES systems first recruit large motor axons instead of smaller axons [116]. Large motor axons recruit muscle fibers that generate large forces but are not resistant to fatigue. This is the opposite of what happens with a natural movement, during which larger fibers are only activated when substantial forces are required. This artificial inverse recruitment rapidly leads to the generation of fatigue, making it technically challenging to produce and sustain large forces [117, 118]. By contrast, SCS does not recruit spinal motoneurons directly. The activation of spinal motoneurons by means of pre-synaptic recruitment of primary afferents leads to a natural recruitment order that is resistant to fatigue and can produce forces capable of sustaining the whole body weight for extended periods of time [72, 90].
Finally, FES applications that rely on the stimulation of motor axons in the peripheral nerves bypass the spinal cord and consequently cannot directly lead to neuroplasticity of spinal circuits. By contrast, such neuroplasticity is believed to mediate the neurological recovery observed during neurorehabilitation facilitated by SCS [119, 120].
3.5.2 Practical Differences: Assistance Versus Therapy
Because of the difficulty to coordinate complex activations of muscles, both implantable and non-invasive FES systems can almost exclusively be used in controlled environments. For this reason, FES therapy is applied during laboratory or clinic sessions of physical therapy. In this sense, an FES system works to assist physical exercise with a therapeutic goal. It is not a wearable assistive system that supports daily living activities in community settings. By contrast, epidural SCS is a fully implantable system that is seamlessly integrated in patient’s lives. Therefore, SCS can be used both to assist physical therapy as well as support activity of daily livings in community settings [34, 39]. Outside the laboratory or clinics, patients are able to use their fully-implanted systems similarly to what patients with Parkinson’s disease do with a DBS implant. In this regard, SCS addresses needs of assistance that cannot be addressed with modern FES devices.
3.6 Conclusion: SCS, a Promising Neuroprosthetic Technology and Neurorehabilitation Therapy After SCI
In this section, we have described how early human studies using tonic SCS in people with SCI laid the groundwork for its subsequent use as a neurorehabilitation technique, in particular its motor-enabling effect which dates back to 1986 [23], and the discovery of stepping-like movements in humans with SCI [27]. Next, the recent re-discovery of the motor-enabling effect of SCS in 2011 led to its integration into intensive rehabilitation programs for volitional control of joint movements and standing [85]. In parallel with these clinical studies, the development of new stimulation paradigms for delivering SCS to the spinal cord, called spatiotemporal SCS, restored locomotion in rodent and non-human primate models of SCI [33, 62]. Finally, we showed that all these scientific and technological advances converged to the demonstration that SCS combined with intensive rehabilitation can support the recovery of voluntary motor control and overground walking in participants with severe and chronic SCI [34, 37, 94]. Current studies now aim at expanding the range of motor and autonomic functions enabled by SCS (e.g., [39, 98]).
Overall, SCS has shown promises both as neuroprosthesis, i.e., an assistive technology that replaces a lost function and provides immediate relief to a particular deficit, and a rehabilitation tool, i.e., a means to train people with SCI to improve their motor functions when combined with intensive physiotherapy (Fig. 18.5). Although both tonic and spatiotemporal SCS bear great potential in terms of neurorehabilitation, spatiotemporal SCS leads to faster functional outcomes in the absence of training, hence a good indication as an effective neuroprosthesis. Another key aspect to consider is the emergence of the long-term recovery of motor functions in the absence of SCS [34, 88], which likely relies on neuroplastic mechanisms triggered by prolonged use of SCS [119, 120].
SCS combined with rehabilitation leads to long-term motor improvements. a Functional outcomes that can be reached after tonic or spatiotemporal SCS combined with intensive rehabilitation over several months. For each picture, the severity of injury of the depicted individual is indicated. Adapted with permission from [34, 37, 94]. b Neurological recovery observed in two subjects with incomplete SCI after six months of intensive rehabilitation combined with SCS. Left: plots reporting changes in 6-min and 10-m walk tests. Tests were performed without body-weight support, following clinical standards. Middle: evaluations of isometric torque production for each joint, quantified before surgery and after rehabilitation without SCS. Right: changes in lower limb motor and sensory scores after rehabilitation. Changes in motor and sensory scores on abbreviated injury scale for all levels below injury are summarized (motor scores; 0: total paralysis, 1: palpable or visible contraction, 2: active movement, gravity eliminated, 3: active movement against gravity, 4: active movement against some resistance, 5: active movement against full resistance). Adapted with permission from [34]. All rights reserved
In conclusion, although clinical studies have uncovered a formidable potential of SCS both for neuroprosthetics and neurorehabilitation, technological developments and larger multicentric clinical trials are required to assess its safety and efficacy for the millions of people living with SCI worldwide.
4 Transcutaneous SCS as a Non-Invasive Complement to Epidural SCS
4.1 Non-Invasive SCS: Stimulating Posterior Roots via Transcutaneous Electrodes
Fifty years after the development of epidural SCS, transcutaneous SCS was developed as a non-invasive method to activate similar neural structures as epidural SCS, i.e., the posterior roots, but from outside the skin [121]. This study was also the first to suggest that “continuous transcutaneous posterior root stimulation represents a novel, non-invasive, neuromodulative approach for individuals with different neurological disorders”. Inspired by an earlier method of high-voltage percutaneous electrical stimulation of the anterior roots to assess afferent peripheral nerve conduction [122, 123], transcutaneous SCS utilizes skin-surface electrodes with one electrode over the spine at the thoracolumbar junction overlying the lumbosacral spinal cord, and a much larger return electrode placed over the lower abdomen or the iliac crests. Computational modeling of epidural SCS has predicted low-threshold sites along proprioceptive fibers at the posterior rootlet-spinal cord interface [31]. In fact, the high angles in fiber orientation and the crossing of the electrical conductivity boundary between the cerebrospinal fluid and the spinal cord make the recruitment of posterior roots possible also by skin-surface electrodes, albeit with lower root specificity than epidural SCS [30].
Because of the high external voltages required to elicit sufficiently strong voltages inside the vertebral bones, transcutaneous SCS requires external stimulators similar to those typically used for traditional functional electrical stimulation (FES) or transcutaneous electrical nerve stimulation (TENS). With this approach, it is possible to directly stimulate proprioceptive afferent fibers within the posterior roots and evoke activity in extremity muscles through spinal reflexes that resemble those elicited by epidural SCS [121, 126, 127]. The sharing of the same low-threshold sites along the posterior root afferent fibers between epidural and transcutaneous SCS found by studies in computational modeling [30, 128], combined with the near-identical reflex responses evoked by both stimulation methods in humans with SCI (Fig. 18.6a), suggest that both epidural and transcutaneous SCS recruit common neural structures through similar mechanisms [46]. However, because of the larger distance from the spine and the reduced focality of the electrical field, the specificity in muscle recruitment is lower with transcutaneous SCS compared to its epidural counterpart [32, 34, 40, 129].
Transcutaneous SCS can enhance motor and locomotor function by recruiting similar structures to epidural SCS. a Post-activation depression and similar response latencies, peak-to-peak amplitudes, and waveforms by paired-pulse transcutaneous and epidural SCS suggest the activation of common neural input structures (predominantly primary afferent fibers within multiple posterior roots) by both techniques. Modified with permission from [46]. b EMG activity and knee angle excursion during voluntary knee flexion attempts with and without tonic transcutaneous SCS. Modified with permission from [124] (Figure published under a Creative Commons license: Figs. 18.6a and b, licensed under CC-BY 4.0). c Modification of muscle activity by 30-Hz transcutaneous SCS during robotic-driven treadmill stepping. Modified with permission from [125], All rights reserved. Note that the EMG activity in quadriceps during SCS suggests an overt activation of rhythm-generating spinal circuits, because the rhythmic bursts are not properly synchronized with stepping. RF, rectus femoris; BF, biceps femoris; TA, tibialis anterior; TS, triceps surae; ST, semitendinosus; MG, medial gastrocnemius; Ham, hamstring; Q quadriceps
4.2 Transcutaneous SCS for Generating Locomotor-Like Movements
Despite reduced specificity, transcutaneous SCS remains an attractive solution for applications that do not seek to achieve highly selective muscle recruitment. Indeed, it does not require any surgical procedure, thereby significantly reducing the risks and costs of the intervention. It also provides a potentially inclusive and affordable solution to obtain, at least in part, motor improvements comparable with those achieved by epidural SCS.
Therefore, multiple studies have investigated the possibility to augment EMG activity and movements (Fig. 18.6b), as well as functional movements during active treadmill stepping in individuals with chronic, motor-incomplete SCI (AIS D) [49, 130, 131]. As a robotic gait orthosis moved the legs of individuals with clinically complete SCI (AIS A) in a walking pattern over a treadmill, a small number of muscles exhibited electromyographic responses [125]. Adding 30 Hz transcutaneous SCS increased the number of activated leg muscles, which had rhythmic activity during the different phases of gait (Fig. 18.6c). Moreover, transcutaneous SCS alone could produce rhythmic activation of leg muscles without the need for triggered stimulation. Four individuals with incomplete SCI (AIS D) were able to voluntarily modify the generated lower limb muscle activity [131, 132]. By consciously modifying their augmented muscle activity according to the gait phase, participants were able to improve the quality of their stepping kinematics, the range of hip and knee movements, and their stride length. As in epidural SCS, turning the stimulation off would immediately cause degradation of walking quality and muscle activity.
The current interpretation of these results is that mechanisms of transcutaneous SCS are at least partially similar to those of epidural SCS. In particular, this interpretation implies that transcutaneous SCS interacts with the flow of step-induced proprioceptive feedback to modulate muscle activity during locomotion through spindle feedback circuits [72]. However, the rhythmic activation without peripheral feedback suggests that transcutaneous SCS could also engage CPGs [27, 70, 71, 133] in addition to proprioceptive feedback circuits [29, 63, 75]. Moreover, a summation process between residual supraspinal inputs (via clinically silent translesional neural connections that survived the injury), and the increased spinal circuits excitability enhanced by transcutaneous SCS, may be a likely explanation for the voluntary control of movement enabled in otherwise paralyzed muscles (Fig. 18.6b) [125].
4.3 Stimulation Parameters for Transcutaneous SCS
Two general methods for stimulation pulse shape configuration are currently used to deliver transcutaneous SCS: conventional and “Russian” current stimulation. Conventional currents consist of mono- or biphasic charge-balanced pulses with rectangular pulses of 1 ms width, delivered at frequencies ranging from about 5 to 100 Hz. Stimulation at 30 Hz is commonly applied to elicit muscle activity or movement, while 50 Hz pulses are used to improve spasticity [130, 134,135,136]. The “Russian current” is composed of 1 ms bursts filled with 10 kHz pulses [137]. Several studies have suggested that it may be possible to use these currents to reduce the potential discomfort of transcutaneous SCS directly below the stimulating surface electrodes [138, 139], while maintaining recruitment of deep neural structures to elicit comparable electrophysiological responses as conventional transcutaneous SCS. The hypothesis is that the temporal summation of graded potentials created by the rapid depolarization and repolarization of high-frequency stimulation may raise the membrane potential of larger fibers enough to be activated without depolarizing unmyelinated C-fibers [140]. Additionally, there is some neurophysiological evidence suggesting C-fibers are less likely to fire in response to high-frequency stimulation compared to large-diameter fibers [141, 142]. However, a recent study comparing the tolerance and responses elicited by the “Russian current” and conventional transcutaneous SCS protocols contradicted this view. While participants could indeed tolerate significantly higher levels of stimulation amplitude with Russian currents, both protocols produced the same amount of discomfort when the amplitudes were adjusted to obtain the same spinally-evoked muscle responses [143]. Therefore, the apparent higher comfortability may be due to the fact that it takes higher currents with 10 kHz burst to accumulate enough charge to stimulate the same deep structures in the spinal cord as with standard protocols.
4.4 Functional Recovery by Long-Term Transcutaneous SCS and Activity-Based Training
Long-term activity-based training with transcutaneous SCS has also been investigated as a method to induce functional recovery in the chronic phase after SCI. An 18-week training strategy involving voluntary modulation of transcutaneous SCS-generated locomotor movements in combination with buspirone, an orally active serotonergic agonist, was tested in five individuals with chronic motor-complete, sensory-incomplete (AIS B) individuals [137]. The ability of individuals to voluntarily modulate the step-like movements induced by transcutaneous SCS improved with training, and participants gained the ability to generate voluntary movements in previously paralyzed muscles, even without stimulation. With stimulation, their motor function was equivalent to that of individuals with AIS C impairments.
In a subsequent single-case study to evaluate the contribution of buspirone [124], a 4-week training paradigm in robot-assisted overground training found that transcutaneous SCS alone or in combination with buspirone, but not buspirone alone, resulted in the lowest dependence on robotic assistance. Moreover, the participant could perform voluntary knee flexion when in the supine position after a 1-week training with transcutaneous SCS alone, but not after 1-week training with the drug alone. Sayenko and colleagues [139] subsequently demonstrated the ability of transcutaneous SCS to enable standing without previous training in 15 individuals with chronic SCI (AIS A, B, C), and a 12-session training program followed by six participants improved their upright balance control and reduced their dependence on external assistance. A post-training clinical evaluation revealed an increase in muscle tone of all participants. Notably, the results of this study are quantitatively similar to those seen in previous investigations with SCS [85, 86].
Although a single session of transcutaneous SCS can facilitate residual voluntary control of single joints and modify the excitability of cortical, corticospinal, and spinal reflexes [144,145,146], it is not sufficient to observe statistically significant improvements in walking performance [145]. Recent studies by several groups highlight the importance of combining SCS therapy with activity-based training to enable consistent improvements in walking [147, 148] and sit-to-stand ability [149].
4.5 Recent Advances to Improve Muscle Recruitment Selectivity in Transcutaneous SCS
Transcutaneous SCS presents a promising non-invasive approach to enable movement, locomotor function, and long-term recovery after SCI. However, the low specificity of transcutaneous SCS in muscle recruitment compared to its epidural counterpart may limit the type of movements and exercises that can be supported and trained during rehabilitation. Nevertheless, recent studies by Calvert, Krenn, and colleagues have demonstrated that positioning surface electrodes in lateralized (Fig. 18.7a) and rostro-caudal locations (Fig. 18.7b) can target specific mediolateral and rostro-caudal spinal cord circuitry toward improved muscle recruitment selectivity [150, 151]. These observations suggest that future advances in electrode configurations, as well as stimulation amplitudes, frequencies, and timing, may further enhance the potential of transcutaneous SCS in non-invasive rehabilitation approaches.
Limitations in muscle recruitment selectivity by transcutaneous SCS can be partially overcome by electrode configuration. a Elicitation of spinal reflexes by lateral and midline transcutaneous SCS. Transcutaneous SCS applied ~2 cm lateral to the midline of the lumbosacral spinal cord can selectively activate ipsilateral spinal sensorimotor circuits and thus ipsilateral lower extremity muscles. Modified with permission from [150]. b Elicitation of posterior root-muscle reflexes by stimulation from different rostro-caudal sites along a multi-electrode array. The multi-electrode array was 152 mm in length with row D positioned over the T11-T12 vertebra. Stimulation of rostral electrodes (row A) elicits responses in quadriceps but not in triceps surae. In contrast, stimulation of the caudal-most row elicits a large response in triceps surae and a small response in quadriceps. Modified with permission from [151]. MG, medial gastrocnemius; MH, medial hamstrings; SOL, soleus; TA, tibialis anterior; VL, vastus lateralis; L, left; Q, quadriceps; TS, triceps surae. All rights reserved
4.6 Conclusion: Transcutaneous SCS is Less Specific Than Epidural SCS but Provides an Inclusive Access to Advanced Healthcare
In summary, transcutaneous SCS is a promising tool to investigate the combination of SCS and rehabilitation for applications that do not require high specificity. However, the high levels of current required to produce substantial muscle activity to support the body weight in people with severe motor paralysis may be uncomfortable and cause large contractions of the back and abdominal muscles. Nevertheless, we believe that transcutaneous SCS should not necessarily be seen in opposition with epidural SCS, but they can be seen as having unique advantages. While less invasive, transcutaneous SCS requires electrode application for every use and requires an external stimulator connected to the electrodes, and may thus suffer from limited repeatability and portability. Therefore, transcutaneous SCS is very well suited to study and perform SCS-enhanced physical training and rehabilitation protocols in the hospital, and perhaps at home with appropriate guidance, but does not currently serve as a neuroprosthetic intervention that sustains motor activity in daily living. Instead, while more invasive, epidural SCS is fully implantable and therefore, by definition portable, thus sustaining motor activities for people with SCI not only in the clinic but in their daily life. These differences should be considered in the evaluation of the risk–benefit ratio, as it is possible that the interactions between descending voluntary input and SCS are limited when the stimulation is turned off. This would reduce the effectiveness of transcutaneous SCS compared to epidural SCS, which can instead be always on. Nevertheless, transcutaneous SCS represents an affordable tool to amplify the outcome of rehabilitation in many centers, for example, in rural areas where access to neurosurgery expertise and expensive invasive devices may be limited.
Change history
31 May 2023
Chapter 18 (Spinal Cord Stimulation to Enable Leg Motor Control and Walking in People with Spinal Cord Injury) was previously published non-open access. It has now been changed to open access under a CC BY 4.0 license and the copyright holder updated to ‘The Author(s)’. The book has also been updated with this change.
References
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–9.
Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155(3758):108–9.
Shealy CN, Taslitz N, Mortimer JT, Becker DP. Electrical inhibition of pain: experimental evaluation. Anesth Analg. 1967;46(3):299–305.
Shealy CN, Mortimer JT, Reswick JB. Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical report. Anesth Analg. 1967;46(4):489.
Krames ES, Peckham PH, Rezai A, Aboelsaad F. Neuromodulation. Section Introduction. Neuromodulation; 2009. p. 3–8.
Cook AW, Weinstein SP. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N Y State J Med. 1973;73(24):2868–72.
Cook AW. Electrical stimulation in multiple sclerosis. Hosp Pract. 1976;11(4):51–8.
Cook AW, Taylor JK, Nidzgorski F. Functional stimulation of the spinal cord in multiple sclerosis. J Med Eng Technol. 1979;3(1):18–23.
Illis LS, Sedgwick EM, Oygar AE, Awadalla MA. Dorsal-column stimulation in the rehabilitation of patients with multiple sclerosis. The Lancet. 1976;307(7974):1383–6.
Illis LS, Sedgwick EM, Tallis RC. Spinal cord stimulation in multiple sclerosis: clinical results. J Neurol Neurosurg Psychiatry. 1980;43(1):1.
Illis LS, Read DJ, Sedgwick EM, Tallis RC. Spinal cord stimulation in the United Kingdom. J Neurol Neurosurg Psychiatry. 1983;46(4):299.
Siegfried J, Lazorthes Y, Broggi G. Electrical spinal cord stimulation for spastic movement disorders. Stereotact Funct Neurosurg. 1981;44(1–3):77–92.
Davis R, Gray E, Kudzma J. Beneficial augmentation following dorsal column stimulation in some neurological diseases. Stereotact Funct Neurosurg. 1981;44(1–3):37–49.
Dooley DM, Sharkey J. Electrostimulation of the nervous system for patients with demyelinating and degenerative diseases of the nervous system and vascular diseases of the extremities. Stereotact Funct Neurosurg. 1977;40(2–4):208–17.
Siegfried J, Krainick J-U, Haas H, Adorjani C, Meyer M, Thoden U. Electrical spinal cord stimulation for spastic movement disorders. Stereotact Funct Neurosurg. 1978;41(1–4):134–41.
Waltz JM, Reynolds LO, Riklan M. Multi-lead spinal cord stimulation for control of motor disorders. Stereotact Funct Neurosurg. 1981;44(4):244–57.
Barolat G, Myklebust JB, Wenninger W. Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Stereotact Funct Neurosurg. 1988;51(1):29–44.
Barolat-Romana G, Myklebust JB, Hemmy DC, Myklebust B, Wenninger W. Immediate effects of spinal cord stimulation in spinal spasticity. J Neurosurg. 1985;62(4):558–62.
Dimitrijevic MM, Illis LS, Nakajima K, Sharkey PC, Sherwood AM. Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Cent Nerv Syst Trauma. 1986;3(2):129–43.
Richardson RR, Cerullo LJ, McLone DG, Gutierrez FA, Lewis V. Percutaneous epidural neurostimulation in modulation of paraplegic spasticity. Acta Neurochir (Wien). 1979;49(3–4):235–43.
Richardson RR, McLone DG. Percutaneous epidural neurostimulation for paraplegic spasticity. Surg Neurol. 1978;9(3):153–5.
Waltz J. Chronic stimulation for motor disorders. Textb Stereotact Funct Neurosurg. 1998.
Barolat G, Myklebust JB, Wenninger W. Enhancement of voluntary motor function following spinal cord stimulation–case study. Appl Neurophysiol. 1986;49(6):307–14.
Waltz JM. Spinal cord stimulation: a quarter century of development and investigation. Stereotact Funct Neurosurg. 1997;69(1–4):288–99.
Bamford JA, Davis SF. Principles of neurophysiological assessment, mapping, and monitoring; 2019. p. 171–9.
Nagel SJ, Wilson S, Johnson MD, Machado A, Frizon L, Chardon MK, et al. Spinal cord stimulation for spasticity: historical approaches, current status, and future directions. Neuromodulation Technol Neural Interface. 2017;20(4):307–21.
Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–76.
Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci. 2007;26(2):275–95.
Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Bassi Luciani L, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33(49):19326–40.
Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehabil Eng. 2010;18(6):637–45.
Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord. 2000;38(8):473–89.
Capogrosso M, Wagner FB, Gandar J, Moraud EM, Wenger N, Milekovic T, et al. Configuration of electrical spinal cord stimulation through real-time processing of gait kinematics. Nat Protoc. 2018;13(9):2031–61.
Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot J-B, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539(7628):284–8.
Wagner FB, Mignardot J-B, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563(7729):65–71.
Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain J Neurol. 2014;137(Pt 5):1394–409.
Calvert JS, Grahn PJ, Strommen JA, Lavrov IA, Beck LA, Gill ML, et al. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J Neurotrauma. 2019;36(9):1451–60.
Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677–82.
Hofstoetter US, Perret I, Bayart A, Lackner P, Binder H, Freundl B, et al. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience. 2021;24(1):101930.
Rowald A, Komi S, Demesmaeker R, Baaklini E, Hernandez-Charpak SD, Paoles E, et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med. 2022.
Greiner N, Barra B, Schiavone G, Lorach H, James N, Conti S, et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat Commun. 2021;12(1):435.
Holsheimer J. Computer modelling of spinal cord stimulation and its contribution to therapeutic efficacy. Spinal Cord. 1998;36(8):531–40.
Lempka SF, Zander HJ, Anaya CJ, Wyant A, Ozinga JG, Machado AG. Patient-specific analysis of neural activation during spinal cord stimulation for pain. Neuromodulation J Int Neuromodulation Soc. 2020;23(5):572–81.
Coburn B, Sin WK. A theoretical study of epidural electrical stimulation of the spinal cord–Part I: Finite element analysis of stimulus fields. IEEE Trans Biomed Eng. 1985;32(11):971–7.
Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117(4):500–44.
Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, et al. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods. 2006;157(2):253–63.
Hofstoetter US, Freundl B, Binder H, Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE. 2018;13(1):1–22.
Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, et al. Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord. 2004;42(7):401–16.
McNeal DR. Analysis of a model for excitation of myelinated nerve. IEEE Trans Biomed Eng. 1976;23(4):329–37.
Hofstoetter US, Danner SM, Freundl B, Binder H, Mayr W, Rattay F, et al. Periodic modulation of repetitively elicited monosynaptic reflexes of the human lumbosacral spinal cord. J Neurophysiol. 2015;114(1):400–10.
Rattay F. Analysis of models for external stimulation of axons. IEEE Trans Biomed Eng BME. 1986;33(10):974–7.
Struijk J, Holsheimer J, Boom H. Excitation of dorsal root fibers in spinal cord stimulation: a theoretical study. IEEE Trans Biomed Eng. 1993;40(7):632–9.
Davidoff RA. The dorsal columns. Neurology. 1989;39(10):1377–85.
Lloyd DPC, McIntyre AK. Dorsal column conduction of group I muscle afferent impulses and their relay through Clarke’s column. J Neurophysiol. 1950;13(1):39–54.
York DH. Somatosensory evoked potentials in man: differentiation of spinal pathways responsible for conduction from the forelimb vs hindlimb. Prog Neurobiol. 1985;25(1):1–25.
Whitsel B, Petrucelli L, Sapiro G. Modality representation in the lumbar and cervical fasciculus gracilis of squirrel monkeys. Brain Res. 1969;15(1):67–78.
Holsheimer J. Which neuronal elements are activated directly by spinal cord stimulation. Neuromodulation J Int Neuromodulation Soc. 2002;5(1):25–31.
Minassian K, McKay WB, Binder H, Hofstoetter US. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics. 2016;13(2):284–94.
Hofstoetter US, Freundl B, Binder H, Minassian K. Recovery cycles of posterior root-muscle reflexes evoked by transcutaneous spinal cord stimulation and of the H reflex in individuals with intact and injured spinal cord. PLoS ONE. 2019;14(12):e0227057.
Magladery J, Teasdall R, Park A, Porter W. Electrophysiological studies of nerve and reflex activity in normal man. V. Excitation and inhibition of two-neurone reflexes by afferent impulses in the same trunk. Bull Johns Hopkins Hosp. 1951;88(6):520–37.
Pierrot-Deseilligny E, Burke D. The circuitry of the human spinal cord. Cambridge: Cambridge University Press; 2012.
Sharpe AN, Jackson A. Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. J Neural Eng. 2014;11(1):016005.
Wenger N, Moraud EM, Gandar J, Musienko P, Capogrosso M, Baud L, et al. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med. 2016;22(2):138–45.
Hofstoetter US, Knikou M, Guertin PA, Minassian K. Probing the human spinal locomotor circuits by phasic step-induced feedback and by tonic electrical and pharmacological neuromodulation. Curr Pharm Des. 2017;23(12):1805–20.
Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol. 1992;38(4):335–78.
Minassian K, Freundl B, Hofstoetter US. The posterior root-muscle reflex. In: Neurophysiology in Neurosurgery. Elsevier; 2020. p. 239–53.
Hofstoetter US, Danner SM, Freundl B, Binder H, Lackner P, Minassian K. Ipsi- and contralateral oligo- and polysynaptic reflexes in humans revealed by low-frequency epidural electrical stimulation of the lumbar spinal cord. Brain Sci. 2021;11(1):112.
Sayenko DG, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol. 2014;111(5):1088–99.
Roby-Brami A, Bussel B. Long-latency spinal reflex in man after flexor reflex afferent stimulation. Brain J Neurol. 1987;110(Pt 3):707–25.
Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17(4):224–38.
Danner SM, Hofstoetter US, Freundl B, Binder H, Mayr W, Rattay F, et al. Human spinal locomotor control is based on flexibly organized burst generators. Brain. 2015;138(3):577–88.
Minassian K, Hofstoetter US, Dzeladini F, Guertin PA, Ijspeert A. The human central pattern generator for locomotion: does it exist and contribute to walking? Neuroscientist. 2017;23(6):649–63.
Formento E, Minassian K, Wagner F, Mignardot JB, Le Goff-Mignardot CG, Rowald A, et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci. 2018;21(12):1728–41.
Minassian K, Hofstoetter US. Spinal cord stimulation and augmentative control strategies for leg movement after spinal paralysis in humans. CNS Neurosci Ther. 2016;22(4):262–70.
Pinter MM, Gerstenbrand F, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control of spasticity. Spinal Cord. 2000;38(9):524–31.
Moraud EM, Capogrosso M, Formento E, Wenger N, DiGiovanna J, Courtine G, et al. Mechanisms underlying the neuromodulation of spinal circuits for correcting gait and balance deficits after spinal cord injury. Neuron. 2016;89(4):814–28.
Talpalar AE, Endo T, Löw P, Borgius L, Hägglund M, Dougherty KJ, et al. Identification of minimal neuronal networks involved in flexor-extensor alternation in the mammalian spinal cord. Neuron. 2011;71(6):1071–84.
Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, et al. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J Neurophysiol. 2006;96(4):1699–710.
Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979;34(2):241–61.
Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull. 1992;28(1):99–105.
Seáñez I, Capogrosso M. Motor improvements enabled by spinal cord stimulation combined with physical training after spinal cord injury: review of experimental evidence in animals and humans. Bioelectron Med. 2021 Oct 28;7(1):16.
Jilge B, Minassian K, Rattay F, Pinter MM, Gerstenbrand F, Binder H, et al. Initiating extension of the lower limbs in subjects with complete spinal cord injury by epidural lumbar cord stimulation. Exp Brain Res. 2004;154(3):308–26.
Carhart MR, He J, Herman R, D’Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2004;12(1):32–42.
Herman R, He J, D’Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40(2):65–8.
Huang H, He J, Herman R, Carhart MR. Modulation effects of epidural spinal cord stimulation on muscle activities during walking. IEEE Trans Neural Syst Rehabil Eng Publ IEEE Eng Med Biol Soc. 2006;14(1):14–23.
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet Lond Engl. 2011;377(9781):1938–47.
Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS ONE. 2015;10(7):e0133998.
Rejc E, Angeli CA, Bryant N, Harkema SJ. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma. 2017;34(9):1787–802.
Rejc E, Angeli CA, Atkinson D, Harkema SJ. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep. 2017;7(1):13476.
Grahn PJ, Lavrov IA, Sayenko DG, Van Straaten MG, Gill ML, Strommen JA, et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92(4):544–54.
Wenger N, Moraud EM, Raspopovic S, Bonizzato M, DiGiovanna J, Musienko P, et al. Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Sci Transl Med. 2014;6(255):255ra133.
Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13(5):561–6.
Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve. 2005;32(3):261–79.
Bonizzato M, Pidpruzhnykova G, DiGiovanna J, Shkorbatova P, Pavlova N, Micera S, et al. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun. 2018;9(1):3015.
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244–50.
Musienko P, van den Brand R, Märzendorfer O, Roy RR, Gerasimenko Y, Edgerton VR, et al. Controlling specific locomotor behaviors through multidimensional monoaminergic modulation of spinal circuitries. J Neurosci Off J Soc Neurosci. 2011;31(25):9264–78.
Radhakrishna M, Steuer I, Prince F, Roberts M, Mongeon D, Kia M, et al. Double-blind, placebo-controlled, randomized phase I/IIa study (safety and efficacy) with Buspirone/Levodopa/Carbidopa (SpinalonTM) in subjects with complete AIS A or motor-complete AIS B spinal cord injury. Curr Pharm Des. 2017;23(12):1789–804.
Gill ML, Linde MB, Hale RF, Lopez C, Fautsch KJ, Calvert JS, et al. Alterations of spinal epidural stimulation-enabled stepping by descending intentional motor commands and proprioceptive inputs in humans with spinal cord injury. Front Syst Neurosci. 2020;14: 590231.
Darrow D, Balser D, Netoff TI, Krassioukov A, Phillips A, Parr A, et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma. 2019;36(15):2325–36.
Peña Pino I, Hoover C, Venkatesh S, Ahmadi A, Sturtevant D, Patrick N, et al. Long-term spinal cord stimulation after chronic complete spinal cord injury enables volitional movement in the absence of stimulation. Front Syst Neurosci. 2020;30(14):35.
Herrity AN, Williams CS, Angeli CA, Harkema SJ, Hubscher CH. Lumbosacral spinal cord epidural stimulation improves voiding function after human spinal cord injury. Sci Rep. 2018;8(1):8688.
Terson de Paleville DGL, Harkema SJ, Angeli CA. Epidural stimulation with locomotor training improves body composition in individuals with cervical or upper thoracic motor complete spinal cord injury: A series of case studies. J Spinal Cord Med. 2019;42(1):32–8.
Aslan SC, Legg Ditterline BE, Park MC, Angeli CA, Rejc E, Chen Y, et al. Epidural spinal cord stimulation of lumbosacral networks modulates arterial blood pressure in individuals with spinal cord injury-induced cardiovascular deficits. Front Physiol. 2018;9:565.
Harkema SJ, Wang S, Angeli CA, Chen Y, Boakye M, Ugiliweneza B, et al. Normalization of Blood Pressure With Spinal Cord Epidural Stimulation After Severe Spinal Cord Injury. Front Hum Neurosci. 2018;12:83.
Squair JW, Gautier M, Mahe L, Soriano JE, Rowald A, Bichat A, et al. Neuroprosthetic baroreflex controls haemodynamics after spinal cord injury. Nature. 2021;590(7845):308–14.
Ganzer PD, Colachis 4th SC, Schwemmer MA, Friedenberg DA, Dunlap CF, Swiftney CE, et al. Restoring the sense of touch using a sensorimotor demultiplexing neural interface. Cell. 2020.
Granat M, Heller B, Nicol D, Baxendale R, Andrews B. Improving limb flexion in FES gait using the flexion withdrawal response for the spinal cord injured person. J Biomed Eng. 1993;15(1):51–6.
Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–60.
Ajiboye AB, Willett FR, Young DR, Memberg WD, Murphy BA, Miller JP, et al. Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration. Lancet Lond Engl. 2017 May 6;389(10081):1821–30.
Kilgore KL, Hoyen HA, Bryden AM, Hart RL, Keith MW, Peckham PH. An implanted upper-extremity neuroprosthesis using myoelectric control. J Hand Surg. 2008;33(4):539–50.
Popovic MR, Popovic DB, Keller T. Neuroprostheses for grasping. Neurol Res. 2002;24(5):443–52.
Ho CH, Triolo RJ, Elias AL, Kilgore KL, DiMarco AF, Bogie K, et al. Functional electrical stimulation and spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25(3):631–54, ix.
Kapadia N, Masani K, Catharine Craven B, Giangregorio LM, Hitzig SL, Richards K, et al. A randomized trial of functional electrical stimulation for walking in incomplete spinal cord injury: effects on walking competency. J Spinal Cord Med. 2014;37(5):511–24.
Barra B, Conti S, Perich MG, Zhuang K, Schiavone G, Fallegger F, et al. Epidural electrical stimulation of the cervical dorsal roots restores voluntary arm control in paralyzed monkeys. 2020;2020.11.13.379750.
Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, et al. Identification of a cellular node for motor control pathways. Nat Neurosci. 2014;17(4):586–93.
Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957;138(2):227–52.
Jones KE, Bawa P. Computer simulation of the responses of human motoneurons to composite 1A EPSPS: effects of background firing rate. J Neurophysiol. 1997;77(1):405–20.
Giat Y, Mizrahi J, Levy M. A musculotendon model of the fatigue profiles of paralyzed quadriceps muscle under FES. IEEE Trans Biomed Eng. 1993;40(7):664–74.
Kirsch R, Rymer W. Neural compensation for muscular fatigue: evidence for significant force regulation in man. J Neurophysiol. 1987;57(6):1893–910.
Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci. 2018;21(4):576–88.
Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron. 2013;80(5):1301–9.
Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve. 2007;35(3):327–36.
de Noordhout AM, Rothwell JC, Thompson PD, Day BL, Marsden CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry. 1988;51(2):174.
Troni W, Bianco C, Moja MC, Dotta M. Improved methodology for lumbosacral nerve root stimulation. Muscle Nerve. 1996;19(5):595–604.
Gad P, Gerasimenko Y, Zdunowski S, Turner A, Sayenko D, Lu DC, et al. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front Neurosci. 2017;11:1–8.
Minassian K, Hofstoetter US, Danner SM, Mayr W, Bruce JA, McKay WB, et al. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil Neural Repair. 2016;30(3):233–43.
Dy CJ, Gerasimenko YP, Edgerton VR, Dyhre-Poulsen P, Courtine G, Harkema SJ. Phase-dependent modulation of percutaneously elicited multisegmental muscle responses after spinal cord injury. J Neurophysiol. 2010;103(5):2808–20.
Hofstoetter US, Minassian K, Hofer C, Mayr W, Rattay F, Dimitrijevic MR. Modification of reflex responses to lumbar posterior root stimulation by motor tasks in healthy subjects. Artif Organs. 2008;32(8):644–8.
Danner SM, Hofstoetter US, Ladenbauer J, Rattay F, Minassian K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A Modeling Study Artif Organs. 2011;35(3):257–62.
de Freitas RM, Sasaki A, Sayenko DG, Masugi Y, Nomura T, Nakazawa K, et al. Selectivity and excitability of upper-limb muscle activation during cervical transcutaneous spinal cord stimulation in humans. J Appl Physiol Bethesda Md 1985. 2021;131(2):746–59.
Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2013;37(2):202–11.
Minassian K, Hofstoetter U, Tansey K, Rattay F, Mayr W, Dimitrijevic M. Transcutaneous stimulation of the human lumbar spinal cord: facilitating locomotor output in spinal cord injury. 2010.
Minassian K, Hofstoetter US, Rattay F. Transcutaneous lumbar posterior root stimulation for motor control studies and modification of motor activity after spinal cord injury. In: Restorative neurology of spinal cord injury. 2011. p. 226–55.
Wiley J. The human central pattern generator for locomotion: does it exist and contribute to walking? (Unpublished). The Neuroscientist. 2010.
Estes SP, Iddings JA, Field-Fote EC. Priming neural circuits to modulate spinal reflex excitability. Front Neurol. 2017;8:17.
Hofstoetter US, Freundl B, Lackner P, Binder H. Transcutaneous spinal cord stimulation enhances walking performance and reduces spasticity in individuals with multiple sclerosis. Brain Sci. 2021;11(4):472.
Hofstoetter US, Freundl B, Danner SM, Krenn MJ, Mayr W, Binder H, et al. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J Neurotrauma. 2020;37(3):481–93.
Gerasimenko Y, Gorodnichev R, Moshonkina T, Sayenko D, Gad P, Edgerton VR. Transcutaneous electrical spinal-cord stimulation in humans. Ann Phys Rehabil Med. 2015;58(4):225–31.
Gad P, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, et al. Non-invasive activation of cervical spinal networks after severe paralysis. J Neurotrauma. 2018;35(18):2145–58.
Sayenko DG, Rath M, Ferguson AR, Burdick JW, Havton LA, Edgerton VR, et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma. 2019;36(9):1435–50.
Ward AR, Chuen WLH. Lowering of sensory, motor, and pain-tolerance thresholds with burst duration using kilohertz-frequency alternating current electric stimulation: part II. Arch Phys Med Rehabil. 2009;90(9):1619–27.
Joseph L, Butera RJ. High-frequency stimulation selectively blocks different types of fibers in frog sciatic nerve. IEEE Trans Neural Syst Rehabil Eng. 2011;19(5):550–7.
Torebjörk HE, Hallin RG. Responses in human A and C fibres to repeated electrical intradermal stimulation 1. J Neurol Neurosurg Psychiatry. 1974;37(6):653.
Manson GA, Calvert JS, Ling J, Tychhon B, Ali A, Sayenko DG. The relationship between maximum tolerance and motor activation during transcutaneous spinal stimulation is unaffected by the carrier frequency or vibration. Physiol Rep. 2020;8(5):e14397.
Kumru H, Flores Á, Rodríguez-Cañón M, Edgerton VR, García L, Benito-Penalva J, et al. Cervical electrical neuromodulation effectively enhances hand motor output in healthy subjects by engaging a use-dependent intervention. J Clin Med. 2021;10(2):195.
Meyer C, Hofstoetter US, Hubli M, Hassani RH, Rinaldo C, Curt A, et al. Immediate effects of transcutaneous spinal cord stimulation on motor function in chronic, sensorimotor incomplete spinal cord injury. J Clin Med. 2020;9(11):3541.
Parhizi B, Barss TS, Mushahwar VK. Simultaneous cervical and lumbar spinal cord stimulation induces facilitation of both spinal and corticospinal circuitry in humans. Front Neurosci. 2021;15:615103.
Estes S, Zarkou A, Hope JM, Suri C, Field-Fote EC. Combined transcutaneous spinal stimulation and locomotor training to improve walking function and reduce spasticity in subacute spinal cord injury: a randomized study of clinical feasibility and efficacy. J Clin Med. 2021;10(6):1167.
Shapkova EY, Pismennaya EV, Emelyannikov DV, Ivanenko Y. Exoskeleton walk training in paralyzed individuals benefits from transcutaneous lumbar cord tonic electrical stimulation. Front Neurosci. 2020;14:416.
Al’joboori Y, Massey SJ, Knight SL, Donaldson N de N, Duffell LD. The effects of adding transcutaneous spinal cord stimulation (tSCS) to sit-to-stand training in people with spinal cord injury: a pilot study. J Clin Med. 2020;9(9):2765.
Calvert JS, Manson GA, Grahn PJ, Sayenko DG. Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans. J Neurophysiol. 2019;122(5):2111–8.
Krenn M, Hofstoetter US, Danner SM, Minassian K, Mayr W. Multi-electrode array for transcutaneous lumbar posterior root stimulation. Artif Organs. 2015;39(10):834–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this chapter
Cite this chapter
Seáñez, I., Capogrosso, M., Minassian, K., Wagner, F.B. (2022). Spinal Cord Stimulation to Enable Leg Motor Control and Walking in People with Spinal Cord Injury. In: Reinkensmeyer, D.J., Marchal-Crespo, L., Dietz, V. (eds) Neurorehabilitation Technology. Springer, Cham. https://doi.org/10.1007/978-3-031-08995-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-08995-4_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08994-7
Online ISBN: 978-3-031-08995-4
eBook Packages: MedicineMedicine (R0)