Abstract
Purpose
The susceptibility vessel sign (SVS) has been described on gradient echo (GRE) magnetic resonance imaging (MRI) in acute ischemic stroke patients by large vessel occlusion. The presence of SVS (SVS+) was associated with treatment outcome and stroke etiology with conflicting results. Based on multicenter data from the THRombectomie des Artères CErebrales (THRACE) study, we aimed to determine if the association between SVS and cardioembolic etiology (CE) was independent of GRE sequence parameters.
Material and Methods
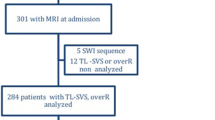
Patients with a pretreatment brain GRE sequence were identified. Logistic regression tested the association between SVS+, CE, time from onset to imaging and GRE sequence parameters (e.g. echo time, voxel size, field strength). We calculated the sensitivity, specificity, positive and negative predictive values (PPV and NPV) for the SVS to predict a stroke from a CE.
Results
An SVS+ was observed in 237 out of 287 (83%) patients. In the univariate analysis, there was a significant association between SVS+ and a CE with an odds ratio (OR) and 95% confidence interval (95% CI) of 2.10 (1.02–4.29), respectively (p = 0.04) but not with GRE sequence parameters. In multivariate analysis, there was an independent relationship between SVS+ and CE (OR [95% CI]: 2.14 [1.02–4.45], p = 0.04). Sensitivity and specificity of SVS+ to predict CE were 0.89 and 0.21, respectively. The PPV and NPV of SVS+ were 0.44 and 0.78, respectively.
Conclusion
The presence of SVS is associated to CE, independent of GRE sequence parameters. While the specificity and the PPV of the sign were low, CE seems less likely in the absence of an SVS.

Similar content being viewed by others
References
Goldstein LB, Jones MR, Matchar DB, Edwards LJ, Hoff J, Chilukuri V, Armstrong SB, Horner RD. Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke. 2001;32:1091–8.
Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CBLM, Dippel DWJ, Siddiqui AH, Khatri P, Baxter B, Nogeuira R, Gounis M, Jovin T, Kallmes DF. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J Neurointerv Surg. 2016;9:529–34.
Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, Zheng DD, Abolian AM, Kim D, Ali LK, Shah SH, Towfighi A, Ovbiagele B, Kidwell CS, Tateshima S, Jahan R, Duckwiler GR, Viñuela F, Salamon N, Villablanca JP, Vinters HV, Marder VJ, Saver JL. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–43.
Boeckh-Behrens T, Schubert M, Förschler A, Prothmann S, Kreiser K, Zimmer C, Riegger J, Bauer J, Neff F, Kehl V, Pelisek J, Schirmer L, Mehr M, Poppert H. The impact of histological clot composition in embolic stroke. Clin Neuroradiol. 2016;26:189–97.
Kim SK, Yoon W, Heo TW, Park MS, Kang HK. Negative susceptibility vessel sign and underlying intracranial atherosclerotic stenosis in acute middle cerebral artery occlusion. AJNR Am J Neuroradiol. 2015;36:1266–71.
Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–93.
Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, de Bruin PC, Mali WP, Velthuis BK. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:e88882.
Sallustio F, Koch G, Di Legge S, Rossi C, Rizzato B, Napolitano S, Samà D, Arnò N, Giordano A, Tropepi D, Misaggi G, Diomedi M, Del Giudice C, Spinelli A, Fabiano S, Stefanini M, Konda D, Reale CA, Pampana E, Simonetti G, Stanzione P, Gandini R. Intra-arterial thrombectomy versus standard intravenous thrombolysis in patients with anterior circulation stroke caused by intracranial arterial occlusions: a single-center experience. J Stroke Cerebrovasc Dis. 2013;22:e323–31.
Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J Neuroradiol. 2015;42:86–92.
Rovira A, Orellana P, Alvarez-Sabín J, Arenillas JF, Aymerich X, Grivé E, Molina C, Rovira-Gols A. Hyperacute ischemic stroke: middle cerebral artery susceptibility sign at echo-planar gradient-echo MR imaging. Radiology. 2004;232:466–73.
Bourcier R, Détraz L, Serfaty JM, Delasalle BG, Mirza M, Derraz I, Toulgoat F, Naggara O, Toquet C, Desal H. MRI interscanner agreement of the association between the susceptibility vessel sign and histologic composition of thrombi. J Neuroimaging. 2017;27:577–82.
Naggara O, Raymond J, Domingo Ayllon M, Al-Shareef F, Touzé E, Chenoufi M, Gerber S, Mellerio C, Zuber M, Meder JF, Mas JL, Oppenheim C. T2* “susceptibility vessel sign” demonstrates clot location and length in acute ischemic stroke. PLoS One. 2013;8:e76727.
Aoki J, Kimura K, Shibazaki K, Sakamoto Y, Saji N, Uemura J. Location of the susceptibility vessel sign on T2*-weighted MRI and early recanalization within 1 hour after tissue plasminogen activator administration. Cerebrovasc Dis Extra. 2013;3:111–20.
Bourcier R, Volpi S, Guyomarch B, Daumas-Duport B, Lintia-Gaultier A, Papagiannaki C, Serfaty JM, Desal H. Susceptibility vessel sign on MRI predicts favorable clinical outcome in patients with anterior circulation acute stroke treated with mechanical thrombectomy. AJNR Am J Neuroradiol. 2015;36:2346–53.
Cho KH, Kim JS, Kwon SU, Cho AH, Kang DW. Significance of susceptibility vessel sign on T2*-weighted gradient echo imaging for identification of stroke subtypes. Stroke. 2005;36:2379–83.
Schellinger PD, Chalela JA, Kang D‑W, Latour LL, Warach S. Diagnostic and prognostic value of early MR Imaging vessel signs in hyperacute stroke patients imaged <3 h and treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2005;26:618–24.
Soize S, Batista AL, Rodriguez Regent C, Trystram D, Tisserand M, Turc G, Serre I, Ben Hassen W, Zuber M, Calvet D, Mas JL, Meder JF, Raymond J, Pierot L, Oppenheim C, Naggara O. Susceptibility vessel sign on T2* magnetic resonance imaging and recanalization results of mechanical thrombectomy with stent retrievers: a multicentre cohort study. Eur J Neurol. 2015;22:967–72.
Legrand L, Naggara O, Turc G, Mellerio C, Roca P, Calvet D, Labeyrie MA, Baron JC, Mas JL, Meder JF, Touzé E, Oppenheim C. Clot burden score on admission T2*-MRI predicts recanalization in acute stroke. Stroke. 2013;44:1878–84.
Kimura K, Sakamoto Y, Iguchi Y, Shibazaki K. Clinical and MRI scale to predict very poor outcome in tissue plasminogen activator patients. Eur Neurol. 2011;65:291–5.
Kang DW, Jeong HG, Kim DY, Yang W, Lee SH. Prediction of stroke subtype and recanalization using susceptibility vessel sign on susceptibility-weighted magnetic resonance imaging. Stroke. 2017;48:1554–9.
Zhang R, Zhou Y, Liu C, Zhang M, Yan S, Liebeskind DS, Lou M. Overestimation of susceptibility vessel sign: a predictive marker of stroke cause. Stroke. 2017;48:1993–6.
Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F; THRACE investigators. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–47.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41.
European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Dávalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millán M, Davis SM, Roy D, Thornton J, Román LS, Ribó M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Cho KY, Miyoshi H, Kuroda S, Yasuda H, Kamiyama K, Nakagawara J, Takigami M, Kondo T, Atsumi T. The phenotype of infiltrating macrophages influences arteriosclerotic plaque vulnerability in the carotid artery. J Stroke Cerebrovasc Dis. 2013;22:910–8.
Shaikh S, Brittenden J, Lahiri R, Brown PA, Thies F, Wilson HM. Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur J Vasc Endovasc Surg. 2012;44:491–7.
Hodel J, Leclerc X, Khaled W, Tamazyan R, Rodallec M, Gerber S, Blanc R, Benadjaoud M, Lambert O, Rabrait C, Zuber M, Rahmouni A, Zins M. Comparison of 3D multi-echo gradient-echo and 2D T2* MR sequences for the detection of arterial thrombus in patients with acute stroke. Eur Radiol. 2014;24:762–9.
Port JD, Pomper MG. Quantification and minimization of magnetic susceptibility artifacts on GRE images. J Comput Assist Tomogr. 2000;24:958–64.
Boeckh-Behrens T, Kleine JF, Zimmer C, Neff F, Scheipl F, Pelisek J, Schirmer L, Nguyen K, Karatas D, Poppert H. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke. 2016;47:1864–71.
Sporns PB, Hanning U, Schwindt W, Velasco A, Minnerup J, Zoubi T, Heindel W, Jeibmann A, Niederstadt TU. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke. 2017;48:2206–10.
Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, Nagatsuka K, Ishibashi-Ueda H, Kira JI, Toyoda K. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke. 2016;47:3035–7.
Ahn SH, Hong R, Choo IS, Heo JH, Nam HS, Kang HG, Kim HW, Kim JH. Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int J Stroke. 2016;11:1036–44.
Qazi EM, Sohn SI, Mishra S, Almekhlafi MA, Eesa M, d’Esterre CD, Qazi AA, Puig J, Goyal M, Demchuk AM, Menon BK. Thrombus characteristics are related to collaterals and angioarchitecture in acute stroke. Can J Neurol Sci. 2015;42:381–8.
Yan S, Liu K, Tong L, Yu Y, Zhang S, Lou M. Different risk factors for poor outcome between patients with positive and negative susceptibility vessel sign. J Neurointerv Surg. 2016;8:1001–5.
Nielsen VG, Kirklin JK, Holman WL, Steenwyk BL. Clot lifespan model analysis of the effects of warfarin on thrombus growth and fibrinolysis: role of contact protein and tissue factor initiation. ASAIO J. 2009;55:33-40.
Yamamoto N, Satomi J, Tada Y, Harada M, Izumi Y, Nagahiro S, Kaji R. Two-layered susceptibility vessel sign on 3‑tesla T2*-weighted imaging is a predictive biomarker of stroke subtype. Stroke. 2015;46:269–71.
Boeckh-Behrens T, Lutz J, Lummel N, Burke M, Wesemann T, Schöpf V, Brückmann H, Linn J. Susceptibility-weighted angiography (SWAN) of cerebral veins and arteries compared to TOF-MRA. Eur J Radiol. 2012;81:1238–45.
Hodel J, Rodallec M, Gerber S, Blanc R, Maraval A, Caron S, Tyvaert L, Zuber M, Zins M. Susceptibility weighted magnetic resonance sequences “SWAN, SWI and VenoBOLD”: technical aspects and clinical applications. J Neuroradiol. 2012;39:71–86.
Park MG, Oh SJ, Baik SK, Jung DS, Park KP. Susceptibility-weighted imaging for detection of thrombus in acute cardioembolic stroke. J Stroke. 2016;18:73–9.
The THRACE trial investigators
are Alain Bonafé (Department of Neuroradiology, Gui de Chauliac Hospital, Montpellier, France), Xavier Leclerc (Department of Radiology, University Hospital of Lille, Lille, France), Nelly Agrinier (Department of Clinical Epidemiology INSERM CIC-EC 1433, University of Lorraine and University Hospital of Nancy, Nancy, France), Serge Bakchine (Department of Neurology, University Hospital of Reims, Reims, France), Flore Baronnet (Stroke Unit, Pitié-Salpêtrière Hospital Group and Paris 6 University—Pierre et Marie Curie, Paris, France), Marine Beaumont (Department of INSERM CIC-IT, University of Lorraine and University Hospital of Nancy, Nancy, France), Yannick Bejot (Department of Neurology, University Hospital of Dijon, Dijon, France), Jerome Berge (Department of Interventional and Diagnostic Neuroradiology, University Hospital of Bordeaux, Bordeaux, France), Marc Bintner (Department of Neuroradiology Sud-Reunion Hospital Group, Saint Pierre, France), Romain Bourcier (Department of Interventional and Diagnostic Neuroradiology, University Hospital of Nantes, Nantes, France), Tae Hee Cho (Department of Neurology, University Hospital of Lyon, Lyon, France), Frédéric Clarencon (Department of Interventional Neuroradiology Pitié-Salpêtrière Hospital Group and Paris 6 University—Pierre et Marie Curie, Paris, France), Julien Cogez (Department of Neurology, University Hospital of Caen, Caen, France), Charlotte Cordonnier (Department of Neurology, University Hospital of Lille, Lille, France), Christian Denier (Department of Neurology, University Hospital of Bicêtre, Le Kremlin-Bicêtre, France), Anne Laure Derelle (Department of Diagnostic and Interventional Neuroradiology, University Hospital of Nancy, Nancy, France), Olivier Detante (Department of Neurology, University Hospital of Grenoble, Grenoble, France), Anthony Faivre (Department of Neurology, Hôpital d’Instruction des Armées, Sainte Anne, Toulon, France), Anne Ferrier, (Department of Neurology, University Hospital Gabriel-Montpied, Clermont-Ferrand, France), Laetitia Gimenez (Department of Neurology, University Hospital of Limoges, Limoges, France), Sophie Godard (Department of Neurology, University Hospital of Angers, Angers, France), Benoit Guillon (Department of Neurology, University Hospital of Nantes, Nantes, France), Emmanuel Houdart (Department of Neuroradiology, University Hospital Lariboisière, Paris, France), Bertrand Lapergue (Department of Neurology, Foch Hospital, Suresnes, France), Mariano Musacchio (Department of Neuroradiology, Pasteur Hospital, Colmar, France), Olivier Naggara (Department of Neuroradiology, Sainte-Anne Hospital and Paris-Descartes University, INSERM U894, Paris, France), Jean Philippe Neau (Department of Neurology, University Hospital of Poitiers, Poitiers, France), Michael Obadia (Department of Neurology, Rothschild Ophthalmological Foundation, Paris, France), Anne Pasco-Papon (Department of Radiology, University Hospital of Angers, Angers, France), Michel Piotin (Department of Interventional Neuroradiology [MP] Rothschild Ophthalmological Foundation, Paris, France), Laurent Pierot (Department of Neuroradiology, University Hospital of Reims, Reims, France), Helene Raoult (Department of Neuroradiology, University Hospital of Rennes, Rennes, France), Sébastien Richard (Department of Neurology University Hospital of Nancy, Nancy, France), Frederic Ricolfi (Department of Neuroradiology, University Hospital of Dijon, Dijon, France), Thomas Ronziere (Department of Neurology, University Hospital of Rennes, Rennes, France), Guillaume Saliou (Department of Neuroradiology, University Hospital of Bicêtre, Le Kremlin-Bicêtre, France), Igor Sibon (Department of Neurology, University Hospital of Bordeaux, Bordeaux, France), Sebastien Soize (Department of Neuroradiology, University Hospital of Reims, Reims, France), Jacques Sedat (Department of Radiology, University Hospital of Nice, Nice, France), Christian Stapf (Department of Neurology, University Hospital Lariboisière, Paris, France), Laurent Suissa (Department of Neurology, University Hospital of Nice, Nice, France), Marie Tisserand (Department of Neuroradiology, Sainte-Anne Hospital and Paris—Descartes University, INSERM U894, Paris, France), Francis Turjman (Department of Interventional Neuroradiology, University Hospital of Lyon, Lyon, France), and Stephane Velasco (Departments of Radiology, University Hospital of Poitiers, Poitiers, France).
Funding
The French Ministry for Health funded this study as part of its 2009 STIC program for the support of costly innovations (grant number 2009 A00753-54).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
R. Bourcier, I. Derraz, B. Delasalle, M. Beaumont, S. Soize, L. Legrand, H. Desal, S. Bracard, O. Naggara and C. Oppenheim declare that they have no competing interests.
Ethical standards
Written informed consent was obtained from all patients or their legal representatives. The study protocol was approved by the Comité de Protection des Personnes III Nord Est Ethics Committee and the research boards of the participating centers.
Additional information
Olivier Naggara and Catherine Oppenheim have equally contributed to this work.
Contributorship Statement
Romain Bourcier had the original idea and co-wrote the manuscript. Catherine Oppenheim and Olivier Naggara co-wrote the manuscript. Marine Beaumont, Sebastien Soize, Laurence Legrand, Serge Bracard, Hubert Desal, Imad Derraz made a critical interpretation of the data and a critical review of the manuscript. Beatrice Delasalle made the statistical interpretation of the data and revised the article.
Data Sharing
Data are available upon request from the corresponding author.
Rights and permissions
About this article
Cite this article
Bourcier, R., Derraz, I., Delasalle, B. et al. Susceptibility Vessel Sign and Cardioembolic Etiology in the THRACE Trial. Clin Neuroradiol 29, 685–692 (2019). https://doi.org/10.1007/s00062-018-0699-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-018-0699-8