Abstract
Objectives
During periodontitis, chronic inflammation triggers soft tissue breakdown, and hyaluronan is degraded into fragments of low molecular weight (LMW-HA). This investigation aimed to elucidate whether LMW-HA fragments with immunogenic potential on T lymphocytes remain in periodontal tissues after periodontal treatment.
Materials and methods
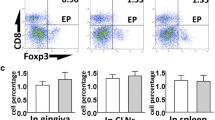
GCF samples were obtained from 15 periodontitis-affected patients and the LMW-HA, RANKL, and OPG levels were analyzed before and after 6 months of periodontal treatment by ELISA. Eight healthy individuals were analyzed as controls. Besides, human T lymphocytes were purified, exposed to infected dendritic cells, and pulsed with LMW-HA. Non-treated T lymphocytes were used as control. The expression levels of the transcription factors and cytokines that determine the Th1, Th17, and Th22 lymphocyte differentiation and function were analyzed by RT-qPCR. Similarly, the expression levels of RANKL and CD44 were analyzed.
Results
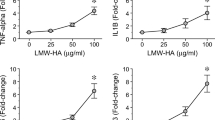
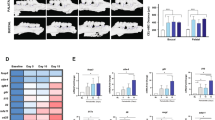
In the GCF samples of periodontitis-affected patients, higher levels of LMW-HA were detected when compared with those of healthy individuals (52.1 ± 15.4 vs. 21.4 ± 12.2, p < 0.001), and these increased levels did not decrease after periodontal therapy (52.1 ± 15.4 vs. 45.7 ± 15.9, p = 0.158). Similarly, the RANKL levels and RANKL/OPG ratios did not change after periodontal therapy. Furthermore, in human T lymphocytes, LMW-HA induced higher expression levels of the Th1, Th17, and Th22-related transcription factors and cytokines, as well as CD44 and RANKL, as compared with non-treated cells.
Conclusions
In some patients, increased levels of LMW-HA persist in periodontal tissues after conventional periodontal therapy, and these remaining LMW-HA fragments with immunostimulatory potential could induce the polarization of a pathologic Th1/Th17/Th22-pattern of immune response on T lymphocytes.
Clinical relevance
The persistence of increased levels of LMW-HA in periodontal tissues after periodontal therapy could favor the recurrence of the disease and further breakdown of periodontal supporting tissues.







Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in this article.
References
Petrey AC, de la Motte CA (2014) Hyaluronan, a crucial regulator of inflammation. Front Immunol 5:101. https://doi.org/10.3389/fimmu.2014.00101
Hernández M, Dutzan N, García-Sesnich J, Abusleme L, Dezerega A, Silva N, González FE, Vernal R, Sorsa T, Gamonal J (2011) Host-pathogen interactions in progressive chronic periodontitis. J Dent Res 90:1164–1170. https://doi.org/10.1177/0022034511401405
Bartold PM, Page RC (1986) The effect of chronic inflammation on gingival connective tissue proteoglycans and hyaluronic acid. J Oral Pathol 15:367–374. https://doi.org/10.1111/j.1600-0714.1986.tb00643.x
Jiang D, Liang J, Noble PW (2011) Hyaluronan as an immune regulator in human diseases. Physiol Rev 91:221–264. https://doi.org/10.1152/physrev.00052.2009
Powell JD, Horton MR (2005) Threat matrix. Low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res 31:207–218. https://doi.org/10.1385/IR:31:3:207
Martins RC, Werneck CC, Rocha LA, Feres-Filho EJ, Silva LC (2003) Molecular size distribution analysis of human gingival glycosaminoglycans in cyclosporin- and nifedipine-induced overgrowths. J Periodontal Res 38:182–189. https://doi.org/10.1034/j.1600-0765.2003.02004.x
Yamalik N, Kilinç K, Çağlayan F, Eratalay K, Çağlayan G (1998) Molecular size distribution analysis of human gingival proteoglycans and glycosaminoglycans in specific periodontal diseases. J Clin Periodontol 25:145–152. https://doi.org/10.1111/j.1600-051x.1998.tb02420.x
Sasaki S, Takeda K, Takewaki M, Ouhara K, Kajiya M, Mizuno N, Fujita T, Kurihara H (2019) BDNF/HMW-HA complex as an adjunct to nonsurgical periodontal treatment of ligature-induced periodontitis in dogs. J Periodontol 90:98–109. https://doi.org/10.1002/JPER.18-0070
Asparuhova MB, Chappuis V, Stähli A, Buser D, Sculean A (2020) Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin Oral Investig 24:3923–3937. https://doi.org/10.1007/s00784-020-03259-8
Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR (2006) Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177:1272–1281. https://doi.org/10.4049/jimmunol.177.2.1272
Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC (2002) Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 195:99–111. https://doi.org/10.1084/jem.20001858
Monasterio G, Guevara J, Ibarra JP, Castillo F, Díaz-Zúñiga J, Alvarez C, Cafferata EA, Vernal R (2019) Immunostimulatory activity of low-molecular-weight hyaluronan on dendritic cells stimulated with Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis. Clin Oral Investig 23:1887–1894. https://doi.org/10.1007/s00784-018-2641-5
Joy RA, Vikkath N, Ariyannur PS (2018) Metabolism and mechanisms of action of hyaluronan in human biology. Drug Metab Pers Ther 33:15–32. https://doi.org/10.1515/dmpt-2017-0031
Schumann J, Stanko K, Schliesser U, Appelt C, Sawitzki B (2015) Differences in CD44 surface expression levels and function discriminates IL-17 and IFN-γ producing helper T cells. PLoS One 10:e0132479. https://doi.org/10.1371/journal.pone.0132479
Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR (1987) Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J Immunol 138:3120–3129
Garlet GP (2010) Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 89:1349–1363. https://doi.org/10.1177/0022034510376402
Díaz-Zúñiga J, Melgar-Rodríguez S, Rojas L, Alvarez C, Monasterio G, Carvajal P, Vernal R (2017) Increased levels of the T-helper 22-associated cytokine (interleukin-22) and transcription factor (aryl hydrocarbon receptor) in patients with periodontitis are associated with osteoclast resorptive activity and severity of the disease. J Periodontal Res 52:893–902. https://doi.org/10.1111/jre.12461
Monasterio G, Budini V, Fernández B, Castillo F, Rojas C, Alvarez C, Cafferata EA, Vicencio E, Cortés BI, Cortez C, Vernal R (2019) IL-22-expressing CD4+AhR+ T lymphocytes are associated with RANKL-mediated alveolar bone resorption during experimental periodontitis. J Periodontal Res 54:513–524. https://doi.org/10.1111/jre.12654
Monasterio G, Castillo F, Ibarra JP, Guevara J, Rojas L, Alvarez C, Fernández B, Agüero A, Betancur D, Vernal R (2018) Alveolar bone resorption and Th1/Th17-associated immune response triggered during Aggregatibacter actinomycetemcomitans-induced experimental periodontitis are serotype-dependent. J Periodontol 89:1249–1261. https://doi.org/10.1002/JPER.17-0563
Plessas A (2014) Nonsurgical periodontal treatment: review of the evidence. Oral Health Dent Manag 13:71–80
Graziani F, Karapetsa D, Alonso B, Herrera D (2017) Nonsurgical and surgical treatment of periodontitis: how many options for one disease? Periodontol 2000 75:152–188. https://doi.org/10.1111/prd.12201
Díaz-Zúñiga J, Yánez JP, Alvarez C, Melgar-Rodríguez S, Hernández M, Sanz M, Vernal R (2014) Serotype-dependent response of human dendritic cells stimulated with Aggregatibacter actinomycetemcomitans. J Clin Periodontol 41:242–251. https://doi.org/10.1111/jcpe.12205
Díaz-Zúñiga J, Melgar-Rodríguez S, Alvarez C, Monasterio G, Benítez A, Ciuchi P, Díaz C, Mardones J, Escobar A, Sanz M, Vernal R (2015) T-lymphocyte phenotype and function triggered by Aggregatibacter actinomycetemcomitans is serotype-dependent. J Periodontal Res 50:824–835. https://doi.org/10.1111/jre.12270
Melgar-Rodríguez S, Díaz-Zúñiga J, Alvarez C, Rojas L, Monasterio G, Carvajal P, Escobar A, Sanz M, Vernal R (2015) Serotype b of Aggregatibacter actinomycetemcomitans increases osteoclast and memory T-lymphocyte activation. Mol Oral Microbiol 31:162–174. https://doi.org/10.1111/omi.12112
Vernal R, Díaz-Zúñiga J, Melgar-Rodríguez S, Pujol M, Díaz-Guerra E, Silva A, Sanz M, Garcia-Sanz JA (2014) Activation of RANKL-induced osteoclasts and memory T lymphocytes by Porphyromonas gingivalis is serotype dependant. J Clin Periodontol 41:451–459. https://doi.org/10.1111/jcpe.12236
Vernal R, León R, Herrera D, Garcia-Sanz JA, Silva A, Sanz M (2008) Variability in the response of human dendritic cells stimulated with Porphyromonas gingivalis or Aggregatibacter actinomycetemcomitans. J Periodontal Res 43:689–697. https://doi.org/10.1111/j.1600-0765.2007.01073.x
Díaz-Zúñiga J, Monasterio G, Alvarez C, Melgar-Rodríguez S, Benítez A, Ciuchi P, García M, Arias J, Sanz M, Vernal R (2015) Variability of the dendritic cell response triggered by different serotypes of Aggregatibacter actinomycetemcomitans or Porphyromonas gingivalis is toll-like receptor 2 (TLR2) or TLR4 dependent. J Periodontol 86:108–119. https://doi.org/10.1902/jop.2014.140326
Liang J, Jiang D, Noble PW (2016) Hyaluronan as a therapeutic target in human diseases. Adv Drug Deliv Rev 97:186–203. https://doi.org/10.1016/j.addr.2015.10.017
Casale M, Moffa A, Vella P, Sabatino L, Capuano F, Salvinelli B, Lopez MA, Carinci F, Salvinelli F (2016) Hyaluronic acid: Perspectives in dentistry. A systematic review. Int J Immunopathol Pharmacol 29:572–582. https://doi.org/10.1177/0394632016652906
Wu M, Cao M, He Y, Liu Y, Yang C, Du Y, Wang W, Gao F (2015) A novel role of low molecular weight hyaluronan in breast cancer metastasis. FASEB J 29:1290–1298. https://doi.org/10.1096/fj.14-259978
Zhang G, Lu R, Wu M, Liu Y, He Y, Xu J, Yang C, Du Y, Gao F (2019) Colorectal cancer-associated ~6 kDa hyaluronan serves as a novel biomarker for cancer progression and metastasis. FEBS J 286:3148–3163. https://doi.org/10.1111/febs.14859
Pogrel MA, Low MA, Stern R (2003) Hyaluronan (hyaluronic acid) and its regulation in human saliva by hyaluronidase and its inhibitors. J Oral Sci 45:85–91. https://doi.org/10.2334/josnusd.45.85
Huber-Lang M, Lambris JD, Ward PA (2018) Innate immune responses to trauma. Nat Immunol 19:327–341. https://doi.org/10.1038/s41590-018-0064-8
Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11:1173–1179. https://doi.org/10.1038/nm1315
Lee-Sayer SS, Dong Y, Arif AA, Olsson M, Brown KL, Johnson P (2015) The where, when, how, and why of hyaluronan binding by immune cells. Front Immunol 6:150. https://doi.org/10.3389/fimmu.2015.00150
Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A (2002) Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J Immunol 169:4322–4331. https://doi.org/10.4049/jimmunol.169.8.4322
Nagy N, Kuipers HF, Marshall PL, Wang E, Kaber G, Bollyky PL (2019) Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol 78-79:292–313. https://doi.org/10.1016/j.matbio.2018.03.022
Chandra J, Kuo PT, Hahn AM, Belz GT, Frazer IH (2017) Batf3 selectively determines acquisition of CD8+ dendritic cell phenotype and function. Immunol Cell Biol 95:215–223. https://doi.org/10.1038/icb.2016.83
Nizzoli G, Krietsch J, Weick A, Steinfelder S, Facciotti F, Gruarin P, Bianco A, Steckel B, Moro M, Crosti M, Romagnani C, Stölzel K, Torretta S, Pignataro L, Scheibenbogen C, Neddermann P, De Francesco R, Abrignani S, Geginat J (2013) Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 122:932–942. https://doi.org/10.1182/blood-2013-04-495424
Tai Y, Wang Q, Korner H, Zhang L, Wei W (2018) Molecular mechanisms of T cells activation by dendritic cells in autoimmune diseases. Front Pharmacol 9:642. https://doi.org/10.3389/fphar.2018.00642
Galandrini R, Galluzzo E, Albi N, Grossi CE, Velardi A (1994) Hyaluronate is costimulatory for human T cell effector functions and binds to CD44 on activated T cells. J Immunol 153:21–31
Lesley J, Howes N, Perschl A, Hyman R (1994) Hyaluronan binding function of CD44 is transiently activated on T cells during an in vivo immune response. J Exp Med 180:383–387. https://doi.org/10.1084/jem.180.1.383
Siegelman MH, DeGrendele HC, Estess P (1999) Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol 66:315–321. https://doi.org/10.1002/jlb.66.2.315
Levesque MC, Haynes BF (1997) Cytokine induction of the ability of human monocyte CD44 to bind hyaluronan is mediated primarily by TNF-alpha and is inhibited by IL-4 and IL-13. J Immunol 159:6184–6194
Gee K, Kozlowski M, Kumar A (2003) Tumor necrosis factor-α induces functionally active hyaluronan-adhesive CD44 by activating sialidase through p38 mitogen-activated protein kinase in lipopolysaccharide-stimulated human monocytic cells. J Biol Chem 278:37275–37287. https://doi.org/10.1074/jbc.M302309200
McAleer JP, Vella AT (2008) Understanding how lipopolysaccharide impacts CD4 T cell immunity. Crit Rev Immunol 28:281–299. https://doi.org/10.1615/critrevimmunol.v28.i4.20
Bastow ER, Byers S, Golub SB, Clarkin CE, Pitsillides AA, Fosang AJ (2008) Hyaluronan synthesis and degradation in cartilage and bone. Cell Mol Life Sci 65:395–413. https://doi.org/10.1007/s00018-007-7360-z
Watanabe T, Takahashi N, Hirabara S, Ishiguro N, Kojima T (2016) Hyaluronan inhibits Tlr-4-dependent RANKL expression in human rheumatoid arthritis synovial fibroblasts. PLoS One 11:e0153142. https://doi.org/10.1371/journal.pone.0153142
Ariyoshi W, Takahashi T, Kanno T, Ichimiya H, Takano H, Koseki T, Nishihara T (2005) Mechanisms involved in enhancement of osteoclast formation and function by low molecular weight hyaluronic acid. J Biol Chem 280:18967–18972. https://doi.org/10.1074/jbc.M412740200
Cao JJ, Singleton PA, Majumdar S, Boudignon B, Burghardt A, Kurimoto P, Wronski TJ, Bourguignon LY, Halloran BP (2005) Hyaluronan increases RANKL expression in bone marrow stromal cells through CD44. J Bone Miner Res 20:30–40. https://doi.org/10.1359/JBMR.041014
Bostanci N, Saygan B, Emingil G, Atilla G, Belibasakis GN (2011) Effect of periodontal treatment on receptor activator of NF-κB ligand and osteoprotegerin levels and relative ratio in gingival crevicular fluid. J Clin Periodontol 38:428–433. https://doi.org/10.1111/j.1600-051X.2011.01701.x
Mio K, Stern R (2002) Inhibitors of the hyaluronidases. Matrix Biol 21:31–37. https://doi.org/10.1016/s0945-053x(01)00185-8
Erickson M, Stern R (2012) Chain gangs: new aspects of hyaluronan metabolism. Biochem Res Int 2012. https://doi.org/10.1155/2012/893947
Acknowledgements
We are grateful to Darna Venegas (Microbiology Laboratory, Faculty of Dentistry, Universidad de Chile) for sharing her expertise on bacterial cultures.
Funding
This investigation has been financially supported by grants FONDECYT 1181780 (RV) and FONDECYT 11190073 (CC), from the Chilean Governmental Agencia Nacional de Investigación y Desarrollo (ANID). GM was a recipient of the Ph.D. Scholarship CONICYT 21170297 from ANID. EAC was a recipient of the Ph.D. scholarship from the School of Graduates of the Faculty of Dentistry, Universidad de Chile.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception and design of the study. FC and GM organized the study, processed the blood samples, performed the cell cultures, and obtained the RNA samples. FC and GM carried out the ELISA experiments and performed the data analysis. JPI, JG, and GM carried out the RT-qPCR experiments and performed the data analysis. FC, EAC, and EV were involved in drafting the manuscript and figures. PC examined the study individuals, performed the periodontal diagnosis and treatment, and critically evaluated and supplemented the manuscript. RV carried out the gingival crevicular fluid sampling. CC and RV designed and implemented the research protocol, performed the data analysis, and prepared the final version of the figures and manuscript for submission. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The performed study design was reviewed and approved by the Ethics Committee for Human Research of Faculty of Dentistry from Universidad de Chile (Protocol #2010/14). Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Castillo, F., Monasterio, G., Ibarra, J.P. et al. Levels of low-molecular-weight hyaluronan in periodontitis-treated patients and its immunostimulatory effects on CD4+ T lymphocytes. Clin Oral Invest 25, 4987–5000 (2021). https://doi.org/10.1007/s00784-021-03808-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-03808-9