Abstract
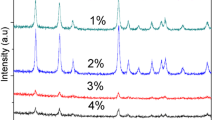
Dysprosium (Dy3+)-doped tin oxide (SnO2) nanoparticles (NPs) have been successfully synthesized using the chemical polymer precursor method. This material blends the holding matrix’s electronic properties with dysprosium’s optical and magnetic properties, making it a promising material for technological applications. X-ray diffraction patterns and the Raman spectra of all NPs indicated the formation of only the SnO2 phase. The decrease in particle size (from ~ 11 to ~ 6 nm) and increase in lattice parameters depending on the Dy content were determined. The latter proves the solid solution between Sn and Dy ions, which is in agreement with the ionic radii mismatch between them. Transmission electron microscopy (TEM) confirms the particle size and size reduction observed through XRD. X-ray photoelectron spectroscopy (XPS) results suggest a change of the oxidation state from Sn4+ to Sn2+with the Dy content, with more Dy3+ than the values accessed from EDS analysis. The latter strongly suggests that the Dy3+ surface gets enriched as the dopant amount increases, driving to the surface passivation of structural defects in good agreement with Raman spectroscopy results. Optical properties show a modest bandgap reduction with the Dy content. Meanwhile, magnetic measurements indicate the coexistence of ferromagnetic and paramagnetic contributions for 1% Dy-doped SnO2 NPs. However, only the paramagnetic contribution is observed after this concentration level. The ferromagnetic contribution detected for lower dopant amounts ( ≤ 1%) has been attributed to the presence of bound magnetic polarons (BMP’s).
Graphical abstract












Similar content being viewed by others
References
Adhikari R, Das A, Karmakar D, Ghatak J (2010) Gd-doped SnO2 nanoparticles: structure and magnetism. J Magn Magn Mater 322:3631–3637
Aragón FH, de Souza PEN, Coaquira JAH, Hidalgo P, Gouvêa D (2012) Spin-glass-like behavior of uncompensated surface spins in NiO nanoparticulated powder. Phys B Condens Matter 407:2601–2605
Aragón FH, Chitta VA, Coaquira JAH, Hidalgo P, Brito HF (2013) Long-range ferromagnetic order induced by a donor impurity band exchange in SnO2:Er3+ nanoparticles. J Appl Phys 114:203902
Aragón F, Villegas-Lelovsky L, Cabral L, Lima M, Aquino J, Mathpal M, Coaquira J, da Silva S, Nagamine L, Parreiras S (2020) Tailoring the physical and chemical properties of Sn 1− x Co x O 2 nanoparticles: an experimental and theoretical approach. Phys Chem Chem Phys 22:3702–3714
Bandyopadhyay A, Modak S, Acharya S, Deb A, Chakrabarti P (2010) Microstructural, magnetic and crystal field investigations of nanocrystalline Dy3+ doped zinc oxide. Solid State Sci 12:448–454
Coelho-Júnior H, Aquino J, Aragón F, Hidalgo P, Cohen R, Nagamine LCCM, Coaquira J, Da Silva S, Brito HFd (2014) Doping effects on the structural, magnetic, and hyperfine properties of Gd-doped SnO 2 nanoparticles. J Nanopart Res 16:2689
Coey J, Venkatesan M, Fitzgerald C (2005) Donor impurity band exchange in dilute ferromagnetic oxides. Nat Mater 4:173–179
Diéguez A, Romano-Rodrìguez A, Vilà A, Morante JR (2001) The complete Raman spectrum of nanometric SnO2 particles. J Appl Phys 90:1550–1557
Duan LB, Chu WG, Yu J, Wang YC, Zhang LN, Liu GY, Liang JK, Rao GH (2008) Structural and magnetic properties of Zn1−xCoxO (0<x⩽0.30) nanoparticles. J Magn Magn Mater 320:1573–1581
Gouvêa D, Pereira GJ, Gengembre L, Steil MC, Roussel P, Rubbens A, Hidalgo P, Castro RH (2011) Quantification of MgO surface excess on the SnO2 nanoparticles and relationship with nanostability and growth. Appl Surf Sci 257:4219–4226
Gubbala S, Chakrapani V, Kumar V, Sunkara MK (2008) Band-edge engineered hybrid structures for dye-sensitized solar cells based on SnO2 nanowires. Adv Funct Mater 18:2411–2418
Hidalgo P, Castro RH, Coelho AC, Gouvêa D (2005) Surface segregation and consequent SO2 sensor response in SnO2− NiO. Chem Mater 17:4149–4153
Hwang S, Kim YY, Lee JH, Seo DK, Lee JY, Cho HK (2012) Irregular electrical conduction types in tin oxide thin films induced by nanoscale phase separation. J Am Ceram Soc 95:324–327
Kwoka M, Ottaviano L, Passacantando M, Santucci S, Czempik G, Szuber J (2005) XPS study of the surface chemistry of L-CVD SnO2 thin films after oxidation. Thin Solid Films 490:36–42
Larson A, Von Dreele R (1994) GSAS: general structural analysis system. Los Alamos National Laboratory, Los Alamos
Lee EJ, Ribeiro C, Giraldi T, Longo E, Leite E, Varela JA (2004) Photoluminescence in quantum-confined SnO 2 nanocrystals: evidence of free exciton decay. Appl Phys Lett 84:1745–1747
Masuda Y, Itoh T, Shin W, Kato K (2015) SnO 2 nanosheet/nanoparticle detector for the sensing of 1-nonanal gas produced by lung cancer. Sci Rep 5:10122
Mofokeng S, Kumar V, Kroon R, Ntwaeaborwa O (2017) Structure and optical properties of Dy3+ activated sol-gel ZnO-TiO2 nanocomposites. J Alloys Compd 711:121–131
Ningthoujam RS, Kulshreshtha S (2009) Nanocrystalline SnO2 from thermal decomposition of tin citrate crystal: luminescence and Raman studies. Mater Res Bull 44:57–62
Pacheco-Salazar DG, Aragón FFH, Villegas-Lelovsky L, Ortiz de Zevallos A, Marques GE, Coaquira JAH (2020) Engineering of the band gap induced by Ce surface enrichment in Ce-doped SnO2 nanocrystals. Appl Surf Sci 527:146794
Pagnier T, Boulova M, Galerie A, Gaskov A, Lucazeau G (2000) Reactivity of SnO2–CuO nanocrystalline materials with H2S: a coupled electrical and Raman spectroscopic study. Sensors Actuators B Chem 71:134–139
Pang C-C, Chen M-H, Lin T-Y, Chou T-C (2001) An amperometric ethanol sensor by using nickel modified carbon-rod electrode. Sensors Actuators B Chem 73:221–227
Pillai SK, Sikhwivhilu LM, Hillie TK (2010) Synthesis, characterization and photoluminescence properties of Dy3+-doped nano-crystalline SnO2. Mater Chem Phys 120:619–624
Shaikh FI, Chikhale LP, Nadargi DY, Mulla IS, Suryavanshi SS (2018) Structural, optical and ethanol sensing properties of Dy-doped SnO2 nanoparticles. J Electron Mater 47:3817–3828
Sturges HA (1926) The choice of a class interval. J Am Stat Assoc 21:65–66
Swart HC (2017) Surface sensitive techniques for advanced characterization of luminescent Materials. Materials 10:906
Tholkappiyan R, Vishista K (2015) Tuning the composition and magnetostructure of dysprosium iron garnets by Co-substitution: an XRD, FT-IR, XPS and VSM study. Appl Surf Sci 351:1016–1024
Van Komen C, Thurber A, Reddy K, Hays J, Punnoose A (2008) Structure–magnetic property relationship in transition metal (M= V, Cr, Mn, Fe, Co, Ni) doped Sn O 2 nanoparticles. J Appl Phys 103:07D141
Venugopal B, Nandan B, Ayyachamy A, Balaji V, Amirthapandian S, Panigrahi BK, Paramasivam T (2014) Influence of manganese ions in the band gap of tin oxide nanoparticles: structure, microstructure and optical studies. RSC Adv 4:6141–6150
Zang X, Li D, Pun E, Lin H (2017) Dy 3+ doped borate glasses for laser illumination. Opt Mater Express 7:2040–2054
Acknowledgements
The authors acknowledge the financial support of CONCYTEC-FONDECYT within the framework of the call E038-01 (contract No. 07-2019-FONDECYT-BM-INC.INV). JAHC want to thank the Brazilian agencies CNPq (Grant Numbers 301455/2017-1, 443652/2018-0) and FAPDF (Grant Number 00193.0000151/2019-20) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 34 kb).
Rights and permissions
About this article
Cite this article
Aquino, J.C.R., Aragón, F.F.H., Pacheco-Salazar, D.G. et al. Influence of Dy doping on the structural, vibrational, optical, electronic, and magnetic properties of SnO2 nanoparticles. J Nanopart Res 23, 90 (2021). https://doi.org/10.1007/s11051-021-05187-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-021-05187-4