Abstract
A comparative analysis of high-Andean Pierina was carried out, including a total of 25 species. Based on morphological evidence, with an emphasis on venation and genitalia and molecular data, using three genetic markers, we confirm the recent subjective synonymy of the generic names Tatochila Butler, 1870, Piercolias, Staudinger, 1894, Hypsochila Ureta, 1955, Infraphulia Field, 1958, Pierphulia Field, 1958, and Theochila Field, 1958 with Phulia Herrich-Schäffer, 1867. Two new species are described, namely Phulia stoddardi Pyrcz & Cerdeña n. sp., from the Andes of Central Peru, which occurs at an unusually high altitude of close to 5000 m a.s.l. in dry puna habitat, and Phulia phantasma Lamas, Willmott & Boyer n. sp., from dry montane forests in northern Peru and southern Ecuador. An overview of high-elevation butterflies is presented, with some discussion on adaptations to this environment.
Similar content being viewed by others
Introduction
As a general rule, species diversity decreases with elevation, with many hypotheses proposed to explain this pattern (Hortal et al. 2013), which is seen in insects, including Lepidoptera, and butterflies in particular (Lawton et al. 1987; McCoy 1990). For individual lineages, however, there are of course exceptions, and the diversity of certain groups is highest at middle elevations, up to the timberline around 3000 m a.s.l. (Brehm et al. 2003; Pyrcz 2004; Pyrcz et al. 2009). However, invariably, once the forest-grassland ecotone is crossed, in both temperate and tropical areas, species diversity dramatically decreases, and it continues to fall with increasing elevation (Pyrcz 2010). Close to the snowline, situated generally around 5000 m a.s.l., very few butterflies exist.
Butterflies occur at or above 5000 m a.s.l. above the sea level in just two regions of the world. In central Asia, several species of Papilionidae, Pieridae, Lycaenidae, Nymphalidae, and Hesperiidae have been reported from altitudes above 5000 m a.s.l. (Tshikolovets 2005; Acharya and Vijayan 2015). Some may be classed as migrants, occasionally observed at elevations well above their normal reproductive areas, such as the renowned migrant Vanessa cardui (Linnaeus, 1758). There are, nevertheless, some Asian species whose entire life cycle occurs within extremely high elevations, mostly in the genus Parnassius Latreille, 1804 (Papilionidae), and it is among them that altitude record-holders can be found, including P. charltonius Gray, [1853] (5400 m a.s.l.), P. stoliczkanus C. Felder & R. Felder, 1865 (5500 m a.s.l.), P. acdestis Grum-Grshimaïlo, 1891 (5600 m a.s.l.), P. maharaja Avinoff, 1916 (5700 m a.s.l.), P. acco Gray, [1853] (5700 m a.s.l.) and P. simo Gray, [1853] (5700 m a.s.l.). A couple of members of the lycaenid genus Polyommatus Latreille, 1804 are known to occur above 5000 m a.s.l., including P. stoliczkana (C. Felder & R. Felder, 1865) and P. (Agriades) lehanus Moore, 1878 (5600 m a.s.l.). Finally, in central Asia, two groups of Pieridae can be found with some frequency above 5000 m a.s.l., including Coliadinae, Colias stoliczkana Moore, 1878 (5600 m a.s.l.), C. fieldii Ménétriés, 1855 (5400 m a.s.l.), C. ladakensis C. Felder & R. Felder, 1865 (5300 m a.s.l.) and C. thrasibulus Fruhstorfer, 1910 (5600 m a.s.l.), as well as Pierinae, (Pontia) Baltia butleri (Moore, 1882) (5500 m a.s.l.), and Pontia (Pontia) callidice (Hübner, [1800]) (5600 m a.s.l.). Some Hesperiidae of the genus Pyrgus Hübner, [1819], and Satyrinae of the genera Paralasa Moore, 1893, Karanasa Moore, 1893 and Paroeneis Moore, 1893 can be found at elevations approaching 5000 m a.s.l. (Tshikolovets 2005). Nonetheless, most, if not all, of the above species have rather broad elevational ranges and also occur at elevations down to 4000 m a.s.l.
The situation is mirrored in the high Andes, notably in the Altiplano of Peru, Bolivia, and Chile. Contrary to Asia, there are no high-elevation Papilionidae, but several species of Hesperiidae (Pyrgus, Hylephila Billberg, 1820), Heliconiinae (Yramea Reuss, 1920), Satyrinae (Argyrophorus Blanchard, 1852, Faunula C. Felder & R. Felder, 1867), and Lycaenidae (Itylos Draudt, 1921, Paralycaeides Nabokov, 1945, Pseudolucia Nabokov, 1945) that are found at 4500–4700 m a.s.l. (Cerdeña et al. 2014; Despland 2014). The picture as regards to Pieridae is somewhat different. Of the Coliadinae, two species (Colias flaveola weberbaueri Strand, 1912, and C. euxanthe hermina (Butler, 1871)) can be observed near 5000 m a.s.l., even if their habitats are generally situated a couple of hundred meters below, but at least 15 species within Pierinae have been recorded at elevations approaching 5000 m a.s.l. (Despland et al. 2012). These Pierinae species have until recently been classified within six genera, most of which, as discussed below, are now regarded as junior subjective synonyms, namely Phulia Herrich-Schäffer, 1867, Pierphulia Field, 1958, Infraphulia Field, 1958, Piercolias Staudinger, 1894, Hypsochila Ureta, 1955, and Tatochila Butler, 1870, although the latter two genera have also been regarded as containing some species down to sea level in Patagonia. Among these, only the former genus Piercolias is confined to elevations around 5000 m a.s.l., representing one of the highest flying butterflies in the world (Oram 2005). The holotype of Piercolias forsteri Field & Herrera, 1977 was collected at 5100 m a.s.l. on the slopes of Cerro Illimani in Bolivia. The record-holder in altitude among Neotropical butterflies is, however, Pierphulia isabelae Field & Herrera, 1977, whose holotype was collected by José Herrera on the volcano Ojos del Salado in Chile at 5400 m a.s.l. (Field and Herrera 1977).
The taxonomy of high-altitude Andean Pieridae was revised by Field (1958), who proposed two new genera and diagnosed all the genera based on morphology (venation, legs, and male and female genitalia). His arrangement was, until recently, considered valid and unchallenged. Shapiro et al. (2007) studied the relationships between high-elevation Andean pierines and Baltia based on two molecular markers (COI and COII), obtaining a monophyletic clade including Pierphulia, Infraphulia, Pierphulia, Tatochila, and Hypsochila, and pointed out that Baltia is not a close relative of these. Wahlberg et al. (2014) proposed a hypothesis of the phylogeny of Pieridae based on five molecular markers, in which they confirmed the monophyly of the above genera. They also included the Brazilian monobasic Theochila Field, 1958, which was placed in a clade with Tatochila and Hypsochila, sister to a clade including four exclusively high-elevation genera, even if all these genera appeared to be very closely related.
In a recent paper, Zhang et al. (2021) used all protein coding genes retrieved from whole-genome shotgun sequences to infer the phylogeny of various butterfly lineages, proposing many new combinations and generic synonyms, and describing several new genera and higher taxa. In their conclusions, with which we concur with some exceptions discussed below, the genera Tatochila, Piercolias, Hypsochila, Theochila, Pierphulia, and Infraphulia were all treated as junior subjective synonyms of Phulia Herrich-Schäffer. Also, in the same paper, for the first time, molecular data were included for Piercolias and the monotypic Reliquia Ackery, 1975, endemic to the Colombian Sierra Nevada de Santa Marta, with the latter newly regarded as a junior subjective synonym of Pontia [Fabricius], 1807.
The discovery of two new species described in this paper, and the uncertainty regarding their generic classification, inspired us to revisit the systematics of high-altitude Neotropical Pierina (with the exception of the loosely related genus Leptophobia, Butler, 1870, which comprises several mid to high-altitude Andean species, belonging to the sister clade of Pierina including non-neotropical genera, such as Pieris Schrank, 1801), with the benefit of both morphological and molecular data and a more comprehensive taxon sample than included in previous studies.
Material and methods
Material
Sampling was carried out with a standard entomological hand-net during several field expeditions co-organized by the Museo de Historia Natural de la Universidad Nacional de San Agustín (MUSA) in Arequipa, Peru in June 2019, and the Nature Education Centre of the Jagiellonian University (CEP-UJ)—formerly Zoological Museum of the Jagiellonian University (MZUJ). Fieldwork was also conducted throughout Peru by GL and collaborators (Museo de Historia Natural de la Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM) over the last 50 years. Material was set and examined, and genital dissections were made mainly in (MUSA) and CEP-UJ, including the collection of Pierre Boyer (PBF). Additional material was examined in the McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, USA (FLMNH), and the collection of Maurizio Bollino, Lecce, Italy (MABO).
Morphology
Male and female abdomens were soaked in 10% KOH solution for 5–10 m a.s.l. min and then cleaned out of soft tissue in water in order to expose genital parts. Female abdomens were stained in chlorazole black to better visualize soft genital tissues. Dissected genitalia were dehydrated by using ethanol 90% and 95% solutions. Head microstructures were examined under an Olympus SZX9 stereo-microscope. Adults were photographed with an Olympus E-500 digital camera and plates were composed with Adobe PhotoShop 8. Nikon digital camera DS-Fi1 and Olympus SZX9 stereo-microscope were used for taking pictures of the dissections, which were then processed in Combine ZP and Corel PHOTO-PAINT X3 programs to enhance clarity and improve quality. Genital dissections were kept in glycerol vials pinned under corresponding specimens. Genital terminology largely follows Razowski (1996) and Klots (1956). The following abbreviations were used: FW, forewing; HW, hindwing; D, dorsum; V, venter.
Molecular analysis
Extraction and amplification of DNA
For molecular analysis, two legs were removed from each of the 52 selected specimens, representing all six previously recognized genera of Pierina that occur in high-Andean habitats (Phulia, Infraphulia, Piercolias, Pierphulia, Tatochila, and Hypsochila), along with the southeast South American Theochila. Legs were detached and preserved in 1 ml of ethanol, prior to mounting of the voucher specimen. DNA was extracted using Macherey–Nagel’s Nucleospin Tissue extraction kit, following the manufacturer’s protocol. Amplification of three separate gene regions, one mitochondrial, and two nuclear was performed in a 20 ul volume. The following primers were used: LCO1490 and HCO2198 (Folmer et al. 1994) for COI (Cytochrome c oxidase subunit I), RpS5f and RpS5r for RpS5 (Ribosomal Protein S5), and Frigga and Burre (Wahlberg and Wheat 2008) for GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The following PCR cycling profile was applied for COI: 95 °C for 5 min, 40 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 1 min 30 s, with a final extension period of 72 °C for 10 m a. s. l.in; and for both nuclear genes: 95 °C for 5 min, 40 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min 30 s, and a final extension period of 72 °C for 10 m a.s.l. in PCR products were sent for purification and sequencing to Macrogen Europe (Amsterdam, Netherlands). Additional sequences of 13 related species were imported from GenBank and BOLD System databases: Phulia nymphula (Blanchard, 1852), Pierphulia rosea (Ureta, 1956), Infraphulia ilyodes (Ureta, 1955), Tatochila autodice (Hübner, 1818), Tatochila mercedis (Eschscholtz, 1821), Tatochila homoeodice (Paravicini, 1910), Tatochila theodice (Boisduval, 1832), Theochila maenacte (Boisduval, 1836), Hypsochila wagenknechti (Ureta, 1938), Hypsochila microdice (Blanchard, 1852), with Ganyra josephina (Godart, 1819), and Ascia monuste (Linnaeus, 1764) as outgroups. Sequences were edited and aligned manually using Bioedit version 7.0.9.0. (Hall 1999). Two datasets were prepared, the first one with COI sequences (74 samples, 612 bp) and the second one including COI, GAPDH, RpS-5, with 60 samples, and 1892 bp in the combined dataset.
Phylogenetic inference
Analyses were carried out for the data set of the three genes combined, as well as for the separate COI data set. Phylogenetic trees were inferred using maximum likelihood (ML). Model selection was determined according to the hierarchical likelihood ratio test as implemented in ModelTest 3.06 (Posada and Crandall 1998), with the starting tree obtained by maximum parsimony to estimate model parameters. The data were analyzed in MEGA X (Kumar et al. 2018) with the GTR + G + I model of sequence evolution and 10,000 bootstrap replicates to estimate branch support.
We performed a divergence time estimation using the StarBEAST 2 v.0.15.5 package (Ogilvie et al. 2017) available in BEAST2 suite v. 2.6.3 (Bouckaert et al. 2019), which implements the Bayesian multispecies coalescent method to infer calibrated species trees (Ogilvie et al. 2017). Substitution models were inferred for each locus with Partitionfinder v.0.1 (Lanfear et al., 2012; Bouckaert & Drummond, 2019). An uncorrelated relaxed clock was used for all loci. We assigned to the mitochondrial COI locus a gene ploidy of 0.5 and to the GAPDH-RpS5 nuclear loci a gene ploidy of 2.0. The Birth–Death model (Gernhard 2008) was used with GrowthRate prior as log-normal (M = 5, S = 2) and relative DeathRate as uniform in [0, 1], while the popMean prior was set to log-normal (M = − 7, S = 2) (Jones et al. 2015). The MCMC chains were run for 70 m a.s.l. million generations and sampled once every 1000 generations. Three independent runs were started and combined with LogCombiner v. 2.5.2 (Bouckaert et al. 2019). The posterior distribution of trees from the analysis was then summarized in TreeAnnotator v. 2.5.2 (Bouckaert et al. 2019) with a selected burn-in of 25% to build the maximum clade credibility tree, and posterior probabilities (pp) were calculated as branch support. Chain convergence was further assessed with Tracer v.1.6 (Rambaut et al. 2014) to confirm sufficient effective sampling size (ESS > 200) (BEAST Developers 2017). Node support is defined as low/weak (pp < 0.50), moderate (pp: 0.50–0.84), high (pp: 0.85–0.94), or strong (pp ≥ 0.95). The trees were visualized using Figtree v.1.4 (Rambaut 2009). To plot the ultrametric tree, the R package Strap (Bell & Lloyd 2015) was used, and the resulting trees were refined in Inkscape 0.92.3 (https://www.inkscape.org).
We used secondary calibrations to time-calibrate the species tree (see Peña et al. 2011; Chazot et al. 2019a, 2019b; Matos-Maraví et al. 2021). The selected calibration points were the crown age of the Pieridae at 76.9–15.9 Mya, and the maximum age of the clade Coliadinae + Pierinae at 66.7 Mya (Chazot et al. 2019a). Furthermore, we also constrained the root with the age of the hypothesized ancestral host plant of the Pierinae, with a maximum age corresponding to the crown age of Brassicaceae to 97 Mya (Foster et al. 2017; Chazot et al. 2019a).
Results
Taxonomy
Phulia stoddardi Pyrcz & Cerdeña n. sp.
Types: HOLOTYPE (male): PERU, Ancash: ruta Conococha – Ocros, Abra, 10°15′22″S,77°15′11″W, 4800 m, 11.vi.2019, J. Farfán and J. Cerdeña, [MUSM]; Paratypes (10 males and 4 females): 1 male and 1 female: same data as the holotype [MUSA]; 5 males and 2 females: Peru, Ancash, paso carretera Conococha–Ocros, 4800–4850 m a.s.l., 13.vi.2019, T. Pyrcz [CEP-UJ] (1 female prep. genit. 1751_18.07.2019/K. Florczyk; prep. mol. CEPUJ 20,190,713, prep. mol. CEPUJ 20,190,714); 4 males and 1 female: Ocros vers Huaraz km 27, 10°15′23″S,77°15′14″W, 4800 m, 11.vi.2019, P. Boyer leg., [PBF].
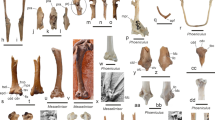
Diagnosis: The new species is closely similar in wing size and shape to species previously assigned to the genus Piercolias, in particular P. cf. forsteri (Fig. 1E–H) from Ticlio, Lima, Peru, and P. huanaco (Staudinger, 1894), although the FW of Phulia stoddardi n. sp. is more elongated with a more convex outer margin. Also, the color pattern is similar to that of P. cf. forsteri, from which it differs in being lighter, with less black on the FWD, and in particular, by the row of subapical black spots which is parallel to the outer margin, whereas in other species previously assigned to Piercolias that same row is sharply displaced basally in cell M3-Cu1. The HWV color is similar to Piercolias, almost patternless but even darker, with a strong greenish suffusion as in P. cf. forsteri, in contrast to the dashed pattern of other high-elevation Pierinae, such as Phulia sensu stricto (Fig. 2A–D) and Infraphulia (Fig. 2E–F). The wing pattern of the species placed until now in Pierphulia (Fig. 2G–H) is, in this respect, intermediate, as it shows darker postdiscal dots on a uniform ground color.
Adults: A Phulia phantasma male holotype, dorsal (Cerro Amancaes); B idem, ventral; C P. phantasma female paratype, dorsal (Llacanora); D idem, ventral; E Phulia (Theochila) maenacte maenacte male (Sao Francisco de Paula), dorsal; F idem, ventral; G Phulia (Theochila) maenacte itatiayae male (Itatiaya), dorsal; H idem, ventral
Description: Adults: MALE (Fig. 1A–B): Head: eyes patchy chestnut and dark brown, naked; palpi 2.5 times the length of head, black, covered with milky white scales; antennae reaching 2/3 of costa, black covered intermittently with white scales, denser on ventral surface of spoon-shaped club. Forewing (length: 17–18 mm) elongated with a convex outer margin and a subacute apex, densely hairy in basal part; fringes long and white. Venation (Fig. 7) characterized by the common stem of forewing M2 and R3+4; R2 arising basally from the stem of R3, not from the same stem as in Piercolias, but similarly to Phulia and Pierphulia; hindwing M1 and M2 arising from the same stem, as in Phulia but contrary to Piercolias, Pierphulia, and Infraphulia. Hindwing oval, densely hairy in basal part; fringes long and white. FWD mostly snow white except for some black scales along costa, a black discal cell end patch, a row of subapical patches, parallel to outer margin, from costa to vein Cu1, and a row of marginal, roughly triangular patches from apex to vein Cu2, gradually smaller. HWD snow white, dusted with some black scales in basal and post-basal area. FWV predominantly snow white, dusted with cream yellow scales along costa, outer margin, apical and subapical area; a faint discal cell black patch; and a series of equally faint subapical patches in cells M1-M2 to M3-Cu1. HWV cream yellow liberally dusted with black scales, denser in basal and post-basal areas, with a series of faint postdiscal black dots, one in every cell. Male genitalia (Fig. 4): similar to other congeners. Uncus short, similar to species formerly placed in Phulia, considerably shorter than tegumen, slightly incised basally; tegumen smooth and thin, uncus short, similar to former Phulia, valva massive with a sharp distal tip, as in former Piercolias, aedeagus short and tubular with a serrate ventral surface and a prominent basal bulbous projection. FEMALE (Fig. 1C–D): sexual dimorphism is slight, the female is somewhat smaller (FW length 15–17 mm), with more prominent black pattern and on the upperside the ground color is richer, milky white. Female genitalia (Fig. 5): Papillae weakly sclerotized and setose; prominent posterior apophyses, genital plates, consisting of large, lateral pockets, inwardly densely setulose, long and scarcely sclerotized ductus bursae except for the initial part, with a prominent accessory pouch, a large corpus bursae with large, double star-like signa at the entrance of ductus and a rudimentary secondary bursa, similar to the species placed previously in Piercolias.
Etymology. This species is dedicated to Terry Stoddard, an amateur lepidopterist from the U.S.A., and co-author of many significant contributions to the knowledge of Nearctic butterflies, in recognition of his excellent collaboration over the years and for sharing his experience on high-elevation butterflies.
Bionomics and distribution
This species has been found so far only at the type locality at the highest part of the road from Conococha to Ocros, at 4800–4950 m a.s.l., on the northern side of the slopes (Fig. 13). Individuals were observed on a dry hillside almost devoid of any vegetation (Fig. 11A–B), except for some sparse “cushion” plants. Two species of Senecio (Asteraceae) were seen, one higher up on the mountain ridge, the other, lower, closer to a bog. Among cushion plants, three species of Asteraceae, Werneria sp., Baccharis sp., and Chaetanthera spp., were identified, as well as Gentiana sedifolia (Gentianaceae) (Fig. 11D–H). No Brassicaceae, a likely hostplant, were observed although, admittedly, due to time constraints, our search was not exhaustive. Individuals of Phulia stoddardi n. sp. remained inactive for most of the morning, until around 11 am when gusty winds stopped blowing. They were observed performing short patrolling flights, very low to the ground, and engaging in energetic interactions with passing individuals. After such interactions, they dropped to the ground and sought shelter or basked on stones with their wings wide open, touching the substrate, in a similar way as with other high-elevation Pierinae. Lateral basking, a posture common among Colias Fabricius, 1807, was not observed. When the temperature decreased, P. stoddardi n. sp. adults were observed seeking shelter by crawling under the rocks, presumably to avoid both freezing and desiccation. Adults were occasionally seen feeding on flowers, although at rest they were more frequently observed sitting on dry, sandy, and rocky slopes. Other species of butterflies seen in the area were exclusively pierids, including occasional passing Colias euxanthe C. Felder & R. Felder, 1865 and some Phulia garleppi Field & Herrera, 1977 (Fig. 11C), with the latter much more common around a bog situated approximately 150 m a.s.l. below the habitat of P. stoddardi n. sp.
Phulia phantasma Lamas, Willmott & Boyer, n. sp.
[Hypsochila n. sp. Lamas (Lamas, 2004: 115, no. 315)]
Types: HOLOTYPE (male): PERU, Ancash, Cerro Amancaes, cerca Santo Toribio, [08°50′S,77°54′W], 3000 m a.s.l., 22.v.[19]80, G. Lamas, MUSM-ENT-006767, [MUSM]; PARATYPES (37 ♂ and 1 ♀): Ancash: 2 males: same data as holotype, MUSM-ENT-006759, 6763, [MUSM]; 2 males: [Cerro] Amancaes, N de Huaylas, [8°50′S,77°54′W], 2900 m a.s.l., 6.v.[19]79, V. Pacheco, MUSM-ENT-006762, 6768, [MUSM]; 1 male: same data, but 3000 m, 8.v.[19]79, MUSM-ENT-006758, [MUSM]; 1 male: Oriente de la Cordillera Negra, C[omunidad] C[ampesina] Shecta, 9°28′06″S,77°35′43″W, 4131 m, 05.ii.2012, B. Medina, [MUSM]; 6 males: road from Bamba, T. Pyrcz [CEP-UJ]; 1 male: same data [PBF]; 4 males: same data, J. Cerdeña & J. Farfan leg., [MUSA]; Cajamarca: 1 male: Cascas-Contumazá p[oste] k[ilometrique] 100, [7°24′35″S,78°48′4″W], 2750 m, 23.iii.2022, P. Boyer, [PBF]; 1 male: Cascas-Contumazá “borne kilometrique” 100, [7°24′66″S,78°48′076″W], 2650–2750 m, 19.vi.2018, [PBF]; 1 male: same data, [MUSA]; 6 males: Chilasque, 06°01′S,79°12′W, 1200 m, 13.vi.2000, G. Lamas, MUSM-ENT-006760, 6761, 6764, 6765, 6770, 6771, [MUSM]; 1 male: same data, except 12.vi.2000, R.K. Robbins, MUSM-ENT-006769, [MUSM]; 1 female: Llacanora, [7°11′S,78°25′W], 2720 m, 19.xii.2006, [R. Vila], [MUSM]; 1 male: Limón-Santa Rosa, 1800–3000 m, i-ii.1998, R. Marx, FLMNH-MGCL-147145, [FLMNH]; 1 male: same data, FLMNH-MGCL-147146, [FLMNH]; 1 male: same data, FLMNH-MGCL-147147, [FLMNH]; 1 male: same data, FLMNH-MGCL-147148; [FLMNH]; 1 male: same data, FLMNH-MGCL-147149; [FLMNH];1 male: same data, FLMNH-MGCL-147150, [FLMNH]; 1 male: vía Celendín-Balsas, El Choloque, [6°51′57″S,78°4′35″W], 1800–2000 m a.s.l., local collectors, vi-vii.2006, [MABO]; Amazonas: 1 male: Chachapoyas, [06°10′S,77°38′W], [2343 m], 1889, M. de Mathan, Ex Oberthür Coll. Brit. Mus. 1927–3., MUSM-ENT-006766, [MUSM]; 1 male: Molinopampa—Granada, [06°23′S,77°26′W, 2800–3000 m a.s.l.], B. Calderón leg., [CEP-UJ]; ECUADOR: Azuay: 1 male: Oña, [03°28′27″S,79°9′32″W], 2200 m a.s.l., 22.iii.[19]65, L.E. Peña, [MUSM].
Diagnosis: Phulia phantasma n. sp. can be recognized from species of similar size previously placed in Hypsochila, Tatochila, or Theochila by its more compact appearance resulting from its less elongate wings, being similar in this respect to some species of Hesperocharis C. Felder, 1862, such as H. marchalii (Guérin-Méneville, [1844]), which is actually syntopic with P. phantasma n. sp. These two species of similar size and flight pattern can be easily confused in the field. Particularly pale individuals of P. phantasma with reduced black in the DFW apex and reduced (or almost absent) dark VHW markings are very similar to some individuals of P. maenacte from southeastern Brazil, but can be readily distinguished (apart from distribution) by having the base of vein R4+5 on the FW much closer to the distal margin than it is to the base of vein M1, whereas in P. maenacte it is approximately equidistant. Otherwise, P. phantasma n. sp. can be distinguished from P. maenacte by the dark scaling that lines the VHW veins broadening and fusing towards the distal margin, rather than tapering or remaining similar in width, and by the presence of a line of dark postdiscal spots in cells M1-Rs to CuA2-2A on the HWV, which may be shaped like distally pointing arrows, or just present as diffuse dark scaling, always absent in P. maenacte but similar to species previously placed in Hypsochila or several species of Tatochila. From the majority of other species of Phulia, P. phantasma n. sp. may be distinguished by the lack of any dark scaling at the end of the FW discal cell, with the dark markings in the distal half of the FW present as a simple black triangular patch which variably fills the apex and may be almost absent. Other superficially similar species (such as those formerly placed in Hypsochila, e.g., P. microdice or P. huemul (Peña, 1964)), have at least the FW discocellular veins lined with black, and a variable line of black FW postdiscal spots.
Description: MALE (Fig. 3A–B): Head: eyes brown, bare, with narrow fringe of white scales at base and a yellow dorso-lateral spot; antennal shaft mixed black and white dorsally (24 antennomeres) with conspicuous rounded club (8 antennomeres) which is pale yellow except for proximal dorsal half which is black; labial palpi white with sparse long, black hair-like scales; top of head and frons white. Thorax: dorsal surface with black scales and long white hair-like scales from sides and near wing bases, ventral surface white, legs with sparse white scaling except for femur with denser white scaling and long white hair-like scales, mid- and hindlegs lacking tibial spurs (present in P. maenacte; Field 1958). Wings: Forewing (length 27–28 mm, n = 5) triangular, hindwing an elongate oval with slightly straighter margin in middle of wing and angled tornus; FW with four radial veins, R4 and R5 fused, with veins R4+5 and R3 originating relatively close to the apex (R4+5 approximately half length of R3+4+5), M2 originating independently of base of M1 + R3+4+5. FWD ground color white, variably present scattered blackish scaling filling apex with uneven basal edge indented in middle of each cell, scattered blackish scaling at very base of wing. HWD similar to forewing except lacking black apical scaling. FWV similar to dorsal surface except lacking dark scaling at wing base and dark apical scaling slightly paler, tinged yellowish, with very indistinct paler yellowish scaling forming intervenal stripes within darker apical area, costa with scattered pale grayish scales. HWV ground color pale yellow, slightly darker orange-yellow anterior of discal cell and more prominently along edge of costa, dark orange-yellow spot at base cell 2A-Cu2; scattered dark grayish scaling lining edges of veins and a forming a “Y”-shaped marking in middle of discal cell, line of indistinct dark gray postdiscal markings in cells 2A-Cu2 to Rs varying from distally pointing arrow shapes to indistinct spots, grayish scaling lining veins broadening and fusing towards margin. Abdomen: dorsal surface black, ventral surface white. Genitalia (Fig. 6): similar in overall form to other members of the genus, for example Phulia wagenknechti (type species of Hypsochila) as figured by Field (1958), notable features include long uncus (similar in length to tegumen), valvae with a blunt distal point, aedeagus of even width, and opening ventrally. In comparison with P. maenacte, there are several differences in the male genitalia, including in P. phantasma n. sp. a much longer uncus, greatly reduced in P. maenacte, and a tapering, downward-pointing aedeagus (flaring and upward-pointing in P. maenacte). FEMALE (Fig. 3C–D): similar to male, except as follows: forewing (length 25 mm, n = 1) and hindwing more rounded, ground color slightly yellowish, dark DFW apical marking more extensive and with intruding pale scaling middle of each cell; ventrally similar to heavily patterned males, FW with trace of indistinct yellowish lines in middle of each cell in apical half, HW with stronger yellowish tinge to pale areas. Genitalia not examined.
Venation pattern of Phulia. A Phulia stoddardi n. sp.: B Phulia (Piercolias) huanaco; C. Phulia (Pierphulia) nysias; D Phulia nymphula; E Phulia garleppi; F Phulia (Infraphulia) madeleinea (B-F redrawn from Field, 1958)
Etymology: The name is a neuter Latin noun in the nominative singular meaning a ghost or phantom, in reference to the pale markings of this species and its mysterious rarity in collections.
Bionomics and distribution: This species is known from a large area of the central tropical Andes, extending from southern Ecuador to central Peru (Fig. 13). It is rare in collections which does not reflect its status in the field. The perceived rarity results from this species occurring in the areas which are seldom visited by lepidopterists, dry Andean valleys, and especially being confused with common white pierids, especially with Hesperocharis marchalii and Leptophobia aripa (Boisduval, 1836). Additionally, P. phantasma n. sp. is a very active, patrolling butterfly, only sporadically seen visiting flowers, such as of yellow and purple Onoseris albicans (Asteraceae) flowers (Fig. 12E), and not seeking humid areas where mud-puddling other pierids are frequently found. The flight is fast and swift. Although it has been recorded from a wide altitudinal range from 1200 to 4131 m, it seems that its optimal elevational range is between 2000 and 2800 m a.s.l. All individuals were collected between December and July, representing the wet season and first half of the dry season.
Molecular phylogeny
Our study provides strong support for the monophyly of the expanded concept (Zhang et al. 2021) of Phulia in both the COI (bs: 94) (Fig. 8) and the concatenated 3-genes tree (bs: 100) (Fig. 9). The internal topology of this large clade differs, however, between analyses based on only COI or on all three genes.
In the COI tree, former Phulia cluster with former Piercolias, Pierphulia, and Infraphulia, as well as with former Hypsochila and three species formerly placed in Tatochila, P. orthodice (Weymer & Maassen 1890), P. mercedis, and P. theodice, and forms a sister clade to P. autodice. Phulia xanthodice (Lucas 1852) and Phulia sp., in turn, form a sister clade to former Phulia and other species plus P. autodice, whereas P. homoeodice is situated on a long external branch relative to all previously mentioned species. Finally, Phulia maenacte and Phulia phantasma cluster together and are sister to all the other taxa. However, the COI tree is not fully resolved because the support values on deeper nodes are low. On the other hand, the support of terminal branches is high but they refer only to single, or exceptionally two species, simply meaning that the species identification based on external morphology fully agrees with barcode data.
In the concatenated tree, the former genera Phulia, Pierphulia, Piercolias, and Infraphulia cluster together in a weakly supported clade (bs: 41), sister to Phulia mercedis + Phulia wagenknechti, but branch support is low (bs: 48). Phulia orthodice is situated in an external position relative to all the abovementioned taxa, on a long branch, but again the common node support is low (bs: 36). Finally, Phulia maenacte and Phulia phantasma n. sp. form a weakly supported clade sister to all the other taxa. As in the COI tree, all the small internal clades composed of one or two species are strongly supported. In the concatenated tree, only a few species of former Tatochila + Hypsochila were included, which hampers understanding of the relationships of species formerly included in these genera in relation to other Pierina, and in particular the species of the Phulia sensu stricto clade. The only interesting noticeable difference is the position of Phulia orthodice as sister to the Phulia s. s. clade on the concatenated tree, and well-rooted in the Phulia sensu stricto clade in the COI tree. Otherwise, the topology of both trees is roughly similar, in particular the sister status of the Phulia phantasma n. sp. + Phulia maenacte clade in relation to the remaining genera.
Our results infer a divergence of the Phulia sensu lato clade from Ganyra josephina in the middle Miocene (Tortonian), ~ 8.8 Mya (4.97–11.8 HPD 95%) (Fig. 10), followed by the subsequent divergence of the two main clades of the Phulia group in the late Pliocene (Piacenzian), ~ 3.3 Mya (2.4–4.2 HPD 95%). The radiation of Phulia sensu lato occurred throughout the Pleistocene, with the divergence of P. maenacte and P. phantasma n. sp. at ~ 2.5 Mya (1.72–3.03 HPD 95%), and that of other clades of Phulia sensu lato slightly later, at ~ 2.1 Mya (1.55–2.51 HPD 95%). The two clades which form the Phulia sensu stricto group originated at ~ 1.8 Mya (1.34–2.07 HPD 95%). In addition, Phulia garleppi diverged from the rest of Phulia sensu lato at ~ 1.9 Mya (1.46–2.18 HPD 95%). The clades (Phulia stoddardi n. sp. + Phulia (“Pierphulia”) sp.) + two species previously associated with Piercolias (P. cf. forsteri + P. coropunae)) diverged at ~ 1.6 Mya (0.52–1.88 HPD 95%). The group comprising two species previously associated with Pierphulia (P. rosea + P. nysias (Weymer & Maassen, 1890)) + Phulia nymphula) separated at ~ 1.4 Mya (0.39–1.62 HPD 95%). The group comprising the species previously placed in Infraphulia (P. ilyodes) + Phulia sensu stricto (P. nannophyes Dyar, 1913 + P. paranympha Staudinger, 1894)) at ~ 1.2 Mya (0.36–1.51 HPD 95%), and the clades ((P. rosea + P. nysias) + P. nymphula) + (P. ilyodes + (P. nannophyes + P. paranympha)) diverged at ~ 1.5 Mya (1.1–1.83 HPD 95%) (Fig. 10).
Habitat of Phulia stoddardi n. sp. in type locality: A Phulia stoddardi n. sp. male in the field, thermoregulating on rocky substrate; B sandy slope with lose boulders where most observations of adults took place, 4850–4900 m; C view on the lake and boggy area at 4700 m, biotope of Phulia garleppi were abundant; D view of the Cordillera Huaywash; E Werneria sp., and Asteraceae sp.; F Asteraceae sp.; G Senecio sp.; H Gentiana sedifolia (photos: P. Boyer)
Habitat of Phulia phantasma n. sp.: A. A cliff above the Contumazá – Cascas road B. Asteraceae plants along the road; C view on the valley of Cascas; D xerophytic vegetation along the Contumazá–Cascas road, 2700 m; E flowers of Onoseris albicans (Asteraceae) frequently visited by numerous species of pierids, including P. phantasma n. sp.; F patches of cloud forest below the Contumazá–Cascas road, 2700 m (photos: P. Boyer)
Discussion
Our results broadly corroborate those of the global genomic study of butterflies of Zhang et al. (2021) and specifically the proposed synonymy of most of the genera (except the loosely related Leptohobia) of montane/temperate Neotropical Pierina with the genus Phulia. The results of that study, however, must be considered preliminary given that although most extant genera were examined, only 11 species were included, and just one or exceptionally two, species per genus. This sample size is potentially too small to reach definitive conclusions on the internal topology of the Neotropical Pierina clade and hence to develop the most appropriate classification. Our study aimed to broaden the taxon sampling, and although it was based on only three genetic markers, it included a much more comprehensive set of taxa, involving 73 samples of almost all known species within this clade (except for two assigned previously to Tatochila and of doubtful specific status (T. inversa Hayward, 1949, and T. mariae Herrera, 1970), and two formerly placed in Hypsochila).
What is clear from both our sets of data is that retaining the systematic status quo is not tenable. This conclusion applies in particular to the retention of Infraphulia, Pierphulia, and Piercolias as separate from Phulia. Such a decision would require the description of more new, species-poor or monobasic genera, for Phulia garleppi and Phulia stoddardi n. sp., without resulting in genera that are morphologically or ecologically reconizable.
The expansion of the former genus Phulia by adding the above three former genera seems appropriate. On the concatenated tree, the clade Phulia sensu stricto plus those three former genera was monophyletic and sister to P. mercedis + P. wagenknechti. However, the clade has a weak node support, and several species assigned before to Tatochila were not included. On the COI tree, on the other hand, the Phulia sensu stricto plus those three former genera was evidently paraphyletic and was recovered as monophyletic only through the addition of several species previously assigned to Tatochila, in particular P. orthodice, P. theodice, P. mercedis, and also both species formerly placed in Hypsochila. Further expanding the genus Phulia to include several considerably larger species included before under Tatochila would require a totally new generic diagnosis. In fact, the genus Phulia, apart from some minor features of venation pattern, was previously differentiated from Tatochila mostly, if not only by, its considerably smaller size (Field 1958).
For similar reasons, maintaining the separate generic status of Tatochila is not defensible. This taxon as formerly conceived is clearly not monophyletic, with some species clustering either with Phulia sensu stricto and relatives, or situated in an external position in relation to Phulia along with some species of former Tatochila, in particular P. orthodice on the concatenated tree, and P. xanthodice, P. sp., and P. homoeodice on the COI tree. In fact, in the light of our molecular study, the retention of Tatochila would require restricting it to the nominate species Synchloë autodice, while removing P. mercedis + former Hypsochila to the expanded Phulia, and describing new genera for P. xanthodice and P. homoeodice. Even acknowledging that T. homoeodice, in particular, is morphologically quite different from other species of former Tatochila, the redefining and retaining of Tatochila would cause significant systematic chaos without resulting in readily recognizable or ecologically compact genera.
The synonymy of all the above genera is further supported by our analysis which indicates a very recent divergence time for the entire group, which would have occurred throughout the Pleistocene, and is correlated with the timing of the Andean orogeny and the availability of high-elevation biomes which developed in the Pliocene some 3–5 Mya (Gregory-Wodzicki 2000). Major climate oscillations in the Pleistocene resulting in subsequent ice ages and warmer interglacials are thought to have promoted habitat turnover and heterogeneity, contributing to faster rates of speciation (Nevado et al. 2018); the timing of the radiation of Phulia is congruent with the massive radiation of páramo plants, such as the megadiverse genus Hypericum, the overwhelming majority of which occurred in the Pleistocene (Nürk et al. 2013).
Finally, the generic classification of Phulia maenacte (Fig. 3E–H) and Phulia phantasma n. sp. would be problematic if attempts were made to retain some of the genera discussed above. These two species diverged from the remaining taxa of the Phulia clade at a somewhat earlier stage, in the Pliocene. The node of the two taxa is weakly supported and their relative branches are long. Theochila is endemic to southeastern Brazil, marginally penetrating into northern Paraguay and Argentina, where it occurs mostly in pampa grasslands from low elevations above the sea level up to 2200 m a.s.l., whereas Phulia phantasma n. sp. is a mid- to high-elevation xeric shrub specialist with adults markedly different from other Andean Pierina. Merging the two species within Theochila would not be justified from any point of view, molecular, biogeographic, or morphological. Their retention as separate entities, on the other hand, would require the description of a second, monobasic genus for Phulia phantasma n. sp., also an unhelpful decision for constructing a useful classification. The external position of Phulia homoeodice in relation to all other taxa of the Phulia complex, including the above two on the COI ML tree, is unexpected. We advocate, at this stage, to maintain Zhang et al.’s (2021) synonymy of all these generic names with Phulia, pending a more comprehensive molecular study of the entire Pierina using substantial DNA sequence data.
The discovery of a new extreme high-elevation species of Phulia in central Peru is a timely reminder that our knowledge of the butterfly fauna of the highest elevations of the Andes remains superficial, and that we still have much to learn about the evolution and biogeography of Andean butterflies. First of all, the elevations at which the species associated previously with Piercolias and the new P. stoddardi n. sp. occur are seldom visited by entomologists, and even when they are, the probabilities of discovering a population of these species remain very low. Their flight period is, as far as is known, confined to the hottest and driest seasons, as for most of the year at 5000 m a.s.l. temperatures remain very low even during the day, with snowfall and constant cloud cover. The difficulty of finding these butterflies on the wing is emphasized by the recent discovery of Phulia (Piercolias) cf. forsteri in Ticlio (Paso Anticona), one of the most frequently visited but seldom collected places in Peru, situated on the main road from Lima to the Amazon basin, and whose Pierinae were briefly studied by Shapiro (1985).
The physiological challenges that this new species needs to overcome, to be able to survive in the climatic conditions at 5000 m a.s.l. in the Andes, are quite unparalleled. Although many Arctic butterflies face very low temperatures, these typically happen during their early stages, in most cases as larvae or pupae, which can find shelter in the soil, leaf litter or under stones, and are therefore much more resistant to extremely low temperatures. In this case, however, the adults also have to survive extreme daily temperature changes. During the hottest days, for example, in Ticlio at 4800 m a.s.l., the temperature can rise to 16–17 °C, and the surface of objects, where the butterflies like to sit, such as rocks, sand, and organic matter, can reach substantially higher temperatures in the strong sun, whereas minimum night temperatures drop to − 20 °C, implying a range of temperature within a 24-h period of around 40 °C. This diurnal range is matched nowhere else, even in Arctic areas or in the mountains of Central Asia. At 5000 m a.s.l. in the Himalayas, maximum day temperatures in July–August are around 12 °C, and night just below 0 °C (www.brittanica.com). Adults of P. stoddardi n. sp. face not only extremely low temperatures but also desiccation since their habitat is very dry and low humidity is reinforced by extreme winds. Gusty winds, which blow for most of the day, can potentially make any insect, and butterfly in particular, flight activity energetically costly or impossible (Mani 1968).
It is also worth noting that most other species of this group, previously assigned to Phulia, Infraphulia, and Pierphulia, are found associated with high-altitude bogs and remain close to humid areas with a predilection for resting on mossy, wet surfaces, or among low grasses, even if they are seen occasionally in drier areas (Shapiro 1985). They can occasionally be observed overflying screes and dry gullies away from marshes, which may be a result of high population densities with some individuals tending to disperse over long distances. However, P. stoddardi n. sp., as well as those species associated before with the genus Piercolias, are highly specialized to very dry, well-exposed sandy slopes with sparse stones and loose cushion plants. This new species also appears to be restricted to a narrow altitude band since all the individuals were observed between 4750 and 5050 m a.s.l., whereas other local species of Phulia or Colias, while occasionally observed near 5000 m a.s.l., have preferred habitats at lower elevations—a phenomenon also found in Central Asia, where some species of Baltia or Pontia may occur locally up to 5400 m a.s.l., but can equally be found down to 3500 m a.s.l.
A detailed study of the ecology and biology of P. stoddardi n. sp. is therefore highly desirable from the perspective of its presumably unique adaptations to its unusual environment. Interestingly, there is apparently no wing size reduction in P. stoddardi n. sp., nor those species associated previously with Piercolias, as a presumed adaptation to extreme altitude environments. On the contrary, these species are of considerably larger size than their close relatives placed previously in Phulia, Infraphulia, and Pierphulia, which also generally occur at lower elevations.
Larval host plants and early stages are known for several species assigned previously to Tatochila and Hypsochila, which feed on Fabaceae (Shapiro 1990, 1991) and Brassicaceae (Shapiro 1985, 1990; Olivares and Hormazábal 1998) but also on Tropaeolaceae (Shapiro 1990). Reports of Tatochila feeding on secondary Cestrum Solanaceae were questioned (Shapiro 1979). Phulia xanthodice is observed frequently in Venezuela and Colombia flying in cabbage fields, which is most probably its substitute host plant. On the other hand, natural hostplants of extreme high-altitude Pierina are unknown. In laboratory conditions Phulia (Pierphulia) rosea was found to lay eggs and feed on the small Lepidium virginicum (L.) Brassicaceae (Shapiro 1986), and the first-instar larvae of Phulia (Infraphulia) ilyodes was reported to accept Brassica nigra (L.) (Shapiro 1985). There is information that, at least in Argentina, Phulia nymphula feeds on Tropaeolum polyphyllum Cav. (Reed 1918), but this is very unlikely in the areas where Phulia stoddardi n. sp. occurs in Peru since no Tropaeolaceae were seen, and these plants usually occur at rather lower elevations. There are very few candidate plants as hosts for Phulia stoddardi n. sp., for example, Draba spp. which are one of the few Brassicaceae known to occur at near 5000 m a.s.l. However, no species of Draba were spotted in the area where adults of Phulia were flying.
An underlying question is: are there more extreme high-altitude butterflies to be discovered in the Andes? Very likely so. The discovery of this new species in the department of Ancash leads to the conclusion that the fauna of extreme altitude pierids is far from being well known and that the exploration of other areas with similar ecological conditions in Peru, Bolivia, or even northern Chile or Argentina could lead to the discovery of yet other fascinating species, or even genera. How high do these species occur? We have found them around 5000 m a.s.l., but, admittedly, higher elevations have not been explored, and there are apparently appropriate habitats at 5300–5500 m a.s.l. in the nearby Cordillera de Huayhuash.
Change history
26 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s13744-023-01045-1
References
Acharya BK, Vijayan L (2015) Butterfly diversity along the elevation gradient of Eastern Himalaya, India. Ecol Res 30:909–919
BEAST Developers (2017) Effective Sample Size (ESS), BEAST Documentation. Available from: https://beast.community/ess_tutorial/ (accessed 7 march 2022)
Bell MA, Lloyd GT (2015) strap: an R package for plotting phylogenies against stratigraphy and assessing their stratigraphic congruence. Palaeontology 58(2):379–389
Bouckaert R, Drummond AJ (2019) bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMN Evol Biol 17(1):1–11
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, du Plessis F, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu CH, Xie D, Zhang C, Stadle T, Drummond AJ (2019) BEAST2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15(4):e1006650. https://doi.org/10.1371/journal.pcbi.1006650
Brehm G, Süßenbach D, Fiedler K (2003) Unique elevational patterns of geometrid moths in an Andean montane rainforest. Ecography 26:456–466
Cerdeña JA, Pyrcz TW, Zacca T (2014) High Andean butterflies from southern Peru, I. Dry puna Satyrinae, with the description of two new taxa and three new records from Peru (Lepidoptera: Nymphalidae). Rev Peru Biol 21(3):213–222
Chazot N, Wahlberg N, Freitas AVL, Mitter C, Labandeira C, Sohn JC, Heikkilä M (2019a) Priors and posteriors in Bayesian timing of divergence analyses: the age of butterflies revisited. Syst Biol 68(5):797–813
Chazot N, Willmott KR, Lamas G, Freitas AVL, Piron-Prunier F, Arias CF, Elias M (2019b) Renewed diversification following Miocene landscape turnover in a Neotropical butterfly radiation. Global Ecol and Biogeo 28(8):1118–1132
Despland E (2014) Butterflies of the high-altitude Atacama Desert: habitat use and conservation. Front Genet 5:1–8
Despland E, Humire R, San Martín S (2012) Species richness and phenology of butterflies along and altitude gradient in the desert of northern Chile. Arc Antarc Alp Res 44(4):423–431
Field WD (1958) A redefinition of the butterfly genera Tatochila, Phulia, Piercolias, and Baltia, with descriptions of related genera and subgenera. Proc U S Natl Mus 108(3396):103–131
Field WD, Herrera L (1977) The Pierid butterflies of the genera Hypsochila Ureta, Phulia Herrich-Schäffer, Infraphulia Field, Pierphulia Field, and Piercolias Staudinger. Smith Contr Zool 232:1–64
Folmer O, Black M, Wr H, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial Cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Foster CSP, Sauquet H, van der Merwe M, McPherson H, Rosette M, Hos YW (2017) Evaluating the impact of genomic data and priors on Bayesian estimates of the angiosperm evolutionary timescale. Syst Biol 66:338–351
Gernhard T (2008) The conditioned reconstructed process. J Theor Biol 253(4):769–778. https://doi.org/10.1016/j.jtbi.2008.04.005
Gregory-Wodzicki KM (2000) Uplift history of the Central and Northern Andes: A Review. Geol Soc Amer Bull 112:1091–1105. https://doi.org/10.1130/0016-7606
Hall TA (1999) Bio Edit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symp Ser 41:95–98
Hortal J, CarrascalLM TKA, Thébault E, Meiri S, Sfenthourakis S (2013) Species richness can decrease with altitude but not with habitat diversity. Proc Natl Acad Sci U S A 110(24):E2149–E2150
Jones G, Aydin Z, Oxelman B (2015) DISSECT: an assignment-free Bayesian discovery method for species delimitation under the multispecies coalescent. Bioinf 31(7):991–998
Klots AB (1956) Lepidoptera. In: Tuxen SL (ed) Taxonomists’ glossary of genitalia in Insects. Munksgaard, Copenhagen, pp 97–110
Kumar S, Stecher G, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549
Lanfear R, Calcott B, Ho SYW, Guindon S (2012) Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1695–1701
Lawton JH, Maccarvin M, Heads PA (1987) Effects of altitude on the abundance and species richness of insect hervibores on bracken. Anim Biol 56:147–160
Mani MS (1968) Ecology and biogeography of high altitude insects. W. Junk, The Hague, p 530
Matos-Maraví P, Wahlberg N, Freitas AVL, DeVries P, Antonelli A, Penz CM (2021) Mesoamerica is a cradle and the Atlantic Forest is a museum of Neotropical butterfly diversity: Insights from the evolution and biogeography of Brassolini (Lepidoptera: Nymphalidae). Biol J Linn Soc 133(3):704–724
McCoy ED (1990) The distribution of insects along elevational gradients. Oikos 58:313–322
Nevado B, Contreraz-Ortiz N, Hughes C, Filatov DA (2018) Pleistocene glacial cycles drive isolation, gene flow and speciation in the high-elevation Andes. New Phytol 219:779–793
Nürk NM, Scheriau C (2013) Madriñán (2013) Explosive radiation in high Andean Hypericum rates of diversification among New World lineages. Front Genet 4:1–14. https://doi.org/10.3389/fgene.2013.00175
Ogilvie HA, Bouckaert RR, Drummond AJ (2017) StarBEAST2 brings faster species tree inference and accurate estimates of substitution rates. Molec Biol Evol 34:2101–2114
Olivares TS, Hormazábal M (1998) Redescripción del adulto, larva, pupa ferata y algunos aspectos biológicos de Tatochila autodice blanchardi [sic] Butler (Lepidoptera: Pieridae). Rev Chil Entomol 25:5–9
Oram DA (2005) The highest flying butterfly in the world? Bull Amateur Entomologist’s Assoc 64(460):84–85
Peña C, Nylin S, Wahlberg N (2011) The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods. Zool J Linn Soc 161:64–87. https://doi.org/10.1111/j.1096-3642.2009.00627.x
Posada D, Crandall K (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818. https://doi.org/10.1093/bioinformatics/14.9.817
Pyrcz TW (2004) Pronophiline butterflies of the highlands of Chachapoyas in northern Peru: faunal survey, diversity and distribution patterns (Lepidoptera, Nymphalidae, Satyrinae). Genus 15(4):455–622
Pyrcz TW (2010) Wybrane zagadnienia z taksonomii, zoogeografii i ewolucji faun górskich na przykładzie grupy modelowej motyli z plemienia Pronophilini (Nymphalidae). Mantis, Olsztyn, p 245
Pyrcz T, Wojtusiak J, Garlacz R (2009) Diversity and distribution patterns of pronophilina butterflies (Lepidoptera: Nymphalidae: Satyrinae) along an altitudinal transect in North-Western Ecuador. Neotrop Entomol 38(6):716–726
Rambaut A (2009) FigTree v1. 3.1. http://tree. bio. ed. ac. uk/software/figtree/.
Rambaut A, Suchard M, Xie D, Drummond AJ (2014) MCMC trace analysis package. Version 1.6. Available from: http://tree.bio.ed.ac.uk/software/tracer/ (accessed 12 January 2022)
Razowski J (1996) Słownik morfologii owadów. PWN, Warszawa, p 431
Reed CS (1918) Breves observaciones acerca de la biología de la Phulia nymphula. Physis (buenos Aires) 4(17):313–314
Shapiro AM (1985) Behavioral and ecological observations of Peruvian high-Andean Pierid butterflies (Lepidoptera). Stud Neotrop Fauna Environ 20(1):1–13
Shapiro AM (1986) The life history of Pierphulia rosea annamariae, an unusual butterfly from the Peruvian Altiplano (Lepidoptera: Pieridae. J N Y Entomol Soc 94(4):536–541
Shapiro AM (1990) The life histories and behavior of the Patagonian-Fuegian white butterflies Hypsochila microdice and H. galactodice (Lepidoptera: Pieridae). J N Y Entomol Soc 98(4):461–473
Shapiro AM (1991) The biology and zoogeography of the legume-feeding Patogonian-Fuegian white butterfly Tatochila theodice (Lepidoptera: Pieridae). J N Y Entomol Soc 99(2):251–260
Shapiro AM, Forister ML, Fordyce JA (2007) Extreme high-altitude Asian and Andean Pierids butterflies are not each other’s closest relatives. Arct Antarct Alp Res 39(1):137–142
Tshikolovets V (2005) The Butterflies of Ladakh (N.-W. India). Vadim Tshikolovets, Brno-Kiev, p 176
Wahlberg N, Wheat CW (2008) Genomic outposts serve the phylogenomic pioneers: designing novel nuclear markers for genomic DNA extractions of Lepidoptera. Syst Biol 57(2):231–242. https://doi.org/10.1080/10635150802033006
Wahlberg N, Rota J, Braby MF, Pierce N, Wheat CW (2014) Revised systematics and higher classification of pierid butterflies (Lepidoptera: Pieridae) based on molecular data. Zool Scr 43(6):641–650
Weymer G, Maassen JP (1890) Lepidopteren gesammelt auf einer Reise durch Colombia, Ecuador, Perú, Brasilien, Argentinien und Bolivien in den Jahren 1868-1877 von Alphons Stübel. Berlin, A. Asher & Co. p. 182
Wheat CW, Vogel H, Wittstock U, Braby MF, Underwood D, Mitchell-Olds T (2007) The genetic basis of a plant–insect coevolutionary key innovation. Proc Natl Acad Sci USA 104(51):20427–20431
Zhang J, Cong Q, Shen J, Opler PA, Grishin NV (2021) Genomics-guided refinement of butterfly taxonomy. Taxonomic Report Int Lepidoptera Survey 9(3):1–54
Acknowledgements
We would like to thank Haydon Warren-Gash (London, UK) for critical reading of the manuscript and Akito Kawahara (FLMNH) and the ButterflyNet Consortium (supported by National Science Foundation DEB-1541500) for providing the sequences of nuclear genes of P. homoeodice.
Nomenclature
ZooBank Registration: urn:lsid:zoobank.org:pub:B5FC1030-BEFF-4F68-B6C5-6B47495BFC2F
Funding
This study was supported in part by the project of the Universidad Nacional de San Agustín de Arequipa (UNSA) with Contract IBA-001–2019-UNSA and by an internal grant of the Institute of Zoology and Biomedical Research of the Jagiellonian University (K/ZDS/007357), and the field work of TP in Peru was supported by the NCN grant Harmonia-10 2018/30/M/NZ8/00293 “Evolutionary biogeography and diversification of the predominantly Andean butterfly subtribe Pronophilina (Nymphalidae, Satyrinae) based on phylogenetic data generated using modern molecular methods.”
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Fieldwork and study site description was performed by T. P., P. B., J. C., J. F., C. F., and G. L. Genitalia dissections, photography and descriptions were performed by K. F., K. W., T. P., G. L., and A. M. DNA extractions, PCR, and electrophoresis were performed by A. Z., whereas bioinformatics and phylogenetic analysis were carried out by O. M., C. F., K. W., and A. Z. The first draft of the manuscript was written by T. P. and K. W., and all the authors commented on previous versions of the manuscript, in particular most of discussion was elaborated by T. P., G. L., P. B., and K. W. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Edited by André VL Freitas.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the ZooBank registration was missing.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pyrcz, T.W., Willmott, K.R., Lamas, G. et al. Considerations on the Systematics of Neotropical Pierina, with the Description of Two New Species of Phulia Herrich-Schäffer from the Peruvian Andes (Lepidoptera: Pieridae, Pierinae, Pierini). Neotrop Entomol 51, 840–859 (2022). https://doi.org/10.1007/s13744-022-00999-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-022-00999-y