Abstract
Objective
This study aimed to describe the results of inspection of clinical trials (CTs) and the feasibility of conducting inspections virtually in Peruvian Social Security hospitals during the pandemic of COVID-19.
Methods
This study described 25 CTs that were inspected during August 2021–November 2021. The data for the variables were obtained from the CT inspection database of the Social Security Sub-directorate of Regulation and Management of Health Research which includes minutes and inspection reports. We describe the characteristics of the CT included and findings during the inspections using relative and absolute frequencies. Likewise, we evaluated the feasibility of virtual inspection through a self-administered questionnaire.
Results
According to the findings of the inspection, 60% of CTs were on biological products, and 60% were focused on infectiology. Additionally, 64% of CTs were implemented in Lima, 52% were conducted in level IV health facilities, and 72% were funded by the pharmaceutical sector. The lack of submission of requested documents (16/25) and inadequate access to the internet (9/15) and source documents (4/15) were the primary observations during the inspection. Regarding the feasibility of virtual supervisions, most interviewees rated their understanding of instruction form as “normal” and its content as “adequate.” Similarly, in the virtual self-assessment matrix, a large proportion of interviewees rated comprehension as “normal” (7/15) and its content as “adequate” (13/15). The quality of the virtual supervision process was 8.6 ± 1.1 on a scale of 1–10.
Conclusion
Discrepancies in records and failure to submit requested documents were the main observations. Most interviewees considered the material to be adequate and gave an overall good rating to the virtual inspection process.
Similar content being viewed by others
Introduction
In Peru, a state of emergency was declared because of the coronavirus disease 2019 (COVID-19) pandemic; hence, on-site activities and—consequently—clinical trial (CT) inspection, that they was face-to-face, were suspended. Due to the increasing number of CTs performed during the pandemic, it was essential to develop compliance strategies for the national and international regulations governing their supervision. To continue the inspection processes during and after the pandemic and to enable decentralized supervision with less time and money spent, the Institute for Health Technology Assessment and Research (IETSI) implemented a new institutional process called “Virtual inspection of CTs.”
A CT is any research conducted on human subjects to determine or confirm the clinical, pharmacological, and pharmacodynamic effects of one or more products under the investigation; it is conducted to detect the adverse reactions as well as to study the absorption, distribution, metabolism, and elimination in order to determine their efficacy or safety [1]. In order to be appropriately conducted, CTs must comply to the ethical and regulatory requirements that ensure the protection of research subjects through their inspection [2]. In Peru, the Institutional Research Ethics Committees (REC) are responsible for inspection of CTs authorized by the “Instituto Nacional de Salud” from Peru (INS)—the governing body for research in the country—from the beginning of the trial to the receipt of the final report, at appropriate intervals depending on the degree of risk posed to the research subjects [1].
In addition, the Institute for Health Technology Assessment and Research (IETSI) within the Peruvian Social Security System (EsSalud) is the decentralized entity that has the function of to inspect the CTs (with main focus on administration) developed at the research centers of the institution in accordance with the policies and norms set out by the Clinical Trials Regulations of the INS and those by the EsSalud itself [3, 4]. However, in their face-to-face format, were suspended because of the social distancing provisions imposed by the Peruvian government following the outbreak of COVID-19 pandemic [3, 5]. Under these circumstances, the INS adapted its technical documents in the context of the pandemic, thereby integrating virtual procedures into its regulations [5]. Because CTs in EsSalud were not inspected by the IETSI since December 2019 and with new CTs developed in the research centers, including those that evaluated drugs against COVID-19, in July 2021, a virtual instrument was built for the inspection of CTs, unprecedented in our country [3].
Although the CT inspections performed by the IETSI cannot replace those performed by the REC, they verify compliance with related regulations as well as with ethical aspects. Thus, they represent a tool that detects possible violations related to the conductance of CTs. Although IETSI previously presented the inspection experience of the CTs developed at our institution, they were face-to-face inspections that were conducted without using the virtual instrument [3, 4]. This study aimed to describe the results of inspection of CTs and the feasibility of conducting inspections virtually in Peruvian Social Security hospitals during the pandemic.
Materials and Methods
This descriptive, cross-sectional, and observational study evaluated the results of the virtual inspection process of the 25 CTs inspected by the IETSI, the regulatory institution of EsSalud, between August 2021 and December 2021. The CTs were conducted by 16 research teams at 10 EsSalud research centers.
Choice of CTs Included in the Study
For the sample, we chose all CTs inspected during the study period. These were chosen from the total number of active CTs in the EsSalud research centers. For sampling, the inspected studies were selected based on the following selection criteria: CT with the largest number of participants allocated in Peru, CT including a vulnerable population, and CT on high-risk or high-cost diseases (e.g., those associated with COVID-19, oncological diseases, or biological therapies). Additionally, the supervision of studies conducted at research facilities with a larger number of authorized and geographically dispersed CTs was prioritized. Furthermore, if randomization was the method used to assign treatments across all inspected CTs. These are the criteria that the IETSI uses to prioritize inspection of CTs in its annual programming.
The information on virtual supervisions was obtained from the IETSI CTs inspections database, which includes all face-to-face inspections before the pandemic and virtual ones during the pandemic. For the present study, only the virtual inspections made with a previously described virtual instrument were included, which included questions about the feasibility of doing the inspections virtually. The information in this database was collected by two investigators (SSO and PHA) separately and verified by a third investigator (DUP).
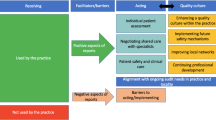
The inspection process has three teams: the administrative coordination team, the evaluation design team, and the final interview team. Likewise, it has five steps.
-
Step 1: Selection of CT to inspect.
-
Step 2: Send the request to the manager of the healthcare network where the research center that runs the CT is located.
-
Step 3: Coordination with the principal investigator via email.
-
- Sending the instructions, the link of the virtual self-assessment matrix, the complementary sheet of the inspection sheet and the list of essential documents (Table 1) during inspection.
-
- Sending the Link of the virtual meeting
-
-
Step 4: Prior to the inspection meeting, the virtual inspection proceeded to the following actions:
-
- Review and verification of all requested documentation.
-
- Coordination meeting of the inspection team and creation of the final guide for the virtual inspection interview.
-
- Creation of the documentary verification mechanism through a group in an application on their phones.
-
-
Step 5: Virtual inspection meeting.
-
Step 6: Sending of the final act of virtual inspection
Aspects Evaluated During Virtual Inspection
The first stage of the virtual inspection started with the development of an instrument whose framework was constructed based on the Good Clinical Practices (GCP) of the Pan American Health Organization, the Clinical Trials Regulations of the INS, and the directive that regulates the development of health research in EsSalud 2019 (the process of which was previously described) [3]. The second stage consisted of the preparation of an instruction manual for the virtual inspection of the CTs developed at EsSalud facilities in a portable document format (PDF) and in the video format for the research teams of the inspected CTs. The instruction manual detailed the whole process of the virtual inspection in a step-by-step manner. These steps started with a notification to the main researcher, followed by the personalized coordination, request for essential documents and self-filling of the virtual inspection instruments, documentation verification, review of documentation, and preparation of the personalized interview guide. Subsequently, the virtual inspection meeting was conducted using the Zoom platform; this was followed by the creation of a group on WhatsApp for requesting planned and unplanned images related to the CTs for supervising and forwarding the final signed minutes. Both access to the WhatsApp group and the Zoom session were managed by the inspection team, which limited access to unidentified personnel.
During the virtual inspection process, from the notification to the main researcher to the recording of the observations described in the final file, the following essential documents related to the conduct of the CT were requested according to the virtual inspection instructions and as needed expand the information if a serious adverse event was reported in the inspected CT.
For each supervised CT, the virtual inspection generates documents, such as applications, the abovementioned essential documents, completed virtual inspection instruments, personalized interview guides, records of the supervision sessions, photographs, and final inspection minutes, which are a part of the IETSI file.
The following variables were included in this study:
-
(A)
General characteristics of inspected CTs
-
–
Study phase: We determined whether the inspected CT belonged to Phase I, II, III, or IV.
-
–
Blinding: This referred to the blinding of the treatment assignment to one or more people involved in the supervised CT, and the type of blinding was open, double, or triple.
-
–
The type of pharmaceutical product: The types of pharmaceutical product were chemical, biological, or both chemical and biological.
-
–
The route of admission: This referred to the way the research product was administered, including oral, subcutaneous, intramuscular, or intravenous routes.
-
–
Specialties: This referred to the medical specialization area of the inspected CT.
-
–
Sponsor: This referred to the organization or person who initiated the study and who had authority and control over the study; these entities included government institutions, pharmaceutical industry, and academic institutions.
-
B)
General characteristics of the implementation of inspected CTs
-
–
Department: This referred to the location of the CT research center, which could be Lima (including the provinces of Lima and Callao) or any of the other 23 provinces of Peru.
-
–
Person responsible for monitoring: This referred to the person who was the link between the sponsor and the main researcher. A contract research organization or an academic institution could be responsible for monitoring.
-
–
The type of institutional REC that approved the CT: This included private REC, EsSalud’s REC, or the National Transitory Committee on Research Ethics (CNTEI).
-
–
The profession of the study coordinator: This referred to the profession of the person designated by the main researcher to perform coordination functions with the research team.
-
C)
Observations found during the virtual inspection of the CTs
-
–
Within the results of the inspections, some observations were found that were grouped as follows
-
–
The presence of SAEs: we determined whether the inspected CT submitted SAEs and whether they were notified to the sponsor and REC/CNTEI.
-
–
Observations related to the crash cart: if present, this included the observations about medications, including those about expired medications, those about medication records, those about medications without expiry dates, and those about medications that were absent from the list.
-
–
Observations related to training: we determined whether there was no record of retraining after deviation from the CT protocol and GCP certificates with no active validity during the conduct of the CT.
-
–
Virtual inspection reprogramming: if the investigation team changed the date it was inspected more than once
-
–
Observations related to the renewal of authorization: we determined whether the Research Center Registration or authorization renewal of the protocol had expired or had expiring authorization (<1 month).
-
–
Observations related to the insurance policy: we determined whether the insurance policy had expired or was not filed.
-
–
Observations related to the reporting of deviations: we determined whether there were discrepancies between the timings of regulatory notifications.
-
–
Observations related to the research product: these included observations related to the storage location of the research product.
-
–
Observations related to the recording of equipment calibrations: we determined whether the calibration was updated.
-
–
Discrepancies in the records: we determined whether the records of the self-assessment tool for the virtual inspection were incomplete, or the recorded data did not match the information in the documentation after verification.
-
–
Documents requested during inspection: we determined whether all requested documents were submitted.
Evaluation of the Feasibility of Virtual Inspection
Additionally, we included perceptions of the principal investigators or study coordinators’ virtual sessions in relation to the understanding and content of the instructions for the virtual inspection instrument and the overall implementation of virtual inspections.
For this variable, a questionnaire was prepared using anonymous Likert-type questions, and it was completed by the main researcher or the study coordinator of the inspected CTs. This questionnaire included a two-part: perception of the material used during CT inspection and perception of the implementation of virtual inspection of CTs. The first part included the perception about Instructions in PDF format, Instructions in video format and Virtual self-assessment matrix. The second part included their perception of the time to schedule virtual inspection, description of difficulties faced during the process, process quality of virtual inspection, adherence to good clinical practices and adherence to bioethical principles.
This questionnaire was created by the authors and evaluated in the pilot test of the virtual inspection instrument in four CTs and their respective research teams with general characteristics like the clinical trials supervised for this study. This questionnaire was self-administered and takes an average of ten minutes.
For the study, the virtual inspection instruments, final minutes of the virtual inspections, and perception questionnaire completed by the inspected research teams were used as sources collected through the IETSI REDcap. The data obtained from individual studies were recorded in a database on Microsoft Excel. Subsequently, we analyzed the distribution of different variables and calculated the relative and absolute frequencies.
The approval of an ethics committee was not required because the units of analysis in this study were not the human subjects but the inspected CTs, except for the perceptions questionnaire. This questionnaire, being anonymous and confidential, was considered low risk and, being in a secondary database, approval by an ethics committee was not requested, as is local policies in some institution in our country. Further, verbal informed consent was obtained to store the information from the supervisions in the IETSI database for reports and research according to the institution's policies.
Results
Of the 25 inspected CTs, most were Phase III (14/25) and double-blind (19/25) trials. The investigational product was a biological product in most trials (15/25), and intravenous administration (10/25) was the most common route of administration. Moreover, approximately 50% of the trials were randomized. The medical specialty to which most of these CTs belonged was infectious diseases (15/25), and the main sponsor was the pharmaceutical industry (18/25) (Table 2).
Most CTs were in Lima (16/25), with a contract research organization (20/25) being primarily responsible for the inspection. Most supervised CTs received CNTEI approval (12/25), and they were conducted at a research center that had a nursing graduate as study coordinator (10/25) (Table 3).
Main Observations Regarding the Feasibility of Virtual Inspection
The main observations of the CTs are presented in Table 4. Regarding the perception of the materials used during the virtual inspection of the CTs, most interviewees rated the understanding of the instructions in PDF as “normal” (10/15) and its content as “adequate” (13/15). Regarding the video format, a large proportion of them rated the understanding of instructions as “easy” (7/15) and its content as “adequate” (11/15). Moreover, regarding the virtual self-assessment matrix, a large proportion of interviewees rated its comprehension as “normal” (7/15) and its content as “adequate” (13/15) (Table 5).
Regarding the perception of the implementation of the virtual inspections, most of the interviewees agreed that the minimum time to schedule a date and time with the principal investigator is 20 days. (13/15). The main difficulties during the virtual inspections were found to be access to the internet (9/15) and source documents (4/15) (Table 6). Regarding the quality of the virtual inspection process, the overall rating on a scale of 1–10 was 8.6 ± 1.1 standard deviation, and regarding the experience in terms of meeting deadlines or times, the overall rating on a scale of 1–10 was 8.8 ± 1.0 standard deviation. Regarding virtual inspection enhancing adherence to bioethical principles (14/15) and supervisions improving adherence to GCP (15/15), most interviewees “agreed” or “strongly agreed” (Table 6).
Discussion
Regarding CT inspection, the Council for International Organizations of Medical Sciences (from their first version in 1982 to their most recent version in 2016) presents guidelines to provide general ethical principles for biomedical research involving human participants [6]. These include recognizing the responsibility of government authorities for ensuring that all studies involving human subjects undergo adequate and timely ethical evaluation as well as monitoring with CT inspection, [6] even in the times of the pandemic.
As mentioned in a previous description on monitored CTs in EsSalud, most of the CTs monitored using our novel instrument were Phase III and double-blind trials, with an investigational product administered intravenously, and they were mostly funded by the pharmaceutical industry. However, in contrast to other study where most research products were chemicals and from the specialty of oncology, [4] most research products in our analysis were biological and from the specialty of infectious diseases. This is related to drugs for SARS-CoV-2. Therefore, it should be noted that the members of the REC should be given the necessary training to assess different designs of biomedical research projects on human subjects, ranging from studies with observational, documentary, field, and stored sample designs to those involving experimental interventions, such as computed tomography scans [7]. Additionally, the COVID-19 outbreak resulted in limitations for the REC’s ability to conduct on-site supervision. Accordingly, in a study conducted in Peru, 114 members of RECs from 18 EsSalud networks were interviewed, and > 60% of them said that it was impossible to supervise CTs in on-site design during the first 2 years of the pandemic [8].
Notably, the CTs on drugs for SARS-CoV-2 followed the national regulations during the pandemic; [5] this implied approval by the CNTEI—the ethics committee in charge of the evaluation of CTs related to the management of COVID-19 [9].
In contrast to a previous study where the main observations were related to the study contract, payment of overhead, and lack of the equipment and supplies necessary to comply with biosafety standards, [4] the main observations of our study could be related to the fact that an institutional innovation process was implemented in the virtual inspection during a state of emergency due to the COVID-19 pandemic. Hence, the discrepancies in the records may be related to the main researchers’ and study coordinators’ process of adaptation to the new formats used by IETSI for the virtual inspection. Nevertheless, the material used for supervision was rated adequate or easy to understand by most interviewees. This implies that we should focus on improving the training of those involved in the virtual inspection for the proper completion of the forms that allow us to assess the development and quality of the data generated from a CT in accordance with GCP, with an emphasis on documentation, national and institutional regulatory requirements, and ethical principles [10].
Similarly, the non-submission of the requested documents may be related to the fact that although a list of essential documents was available, further supporting documents were requested on an unannounced basis during the inspection. Traditionally, CT supervisions in Peru included sessions of 1 day, and although the virtual inspection process is not specifically limited to the virtual session, this finding relates to what is noted in the final virtual inspection minutes, with observations arranged for recording within a maximum period of 48 h. In other words, these missing documents were sent via e-mail to the inspection team within the established deadline.
Regarding the identified difficulties, the lack of access to source documents was most common, with one of the most important difficulties being the lack of access to medical records for verifying the information. As previously mentioned, this finding is related to the fact that most CTs were performed in the context of the pandemic, and due to biosecurity regulations, access to physical documents was restricted or there were difficulties in verifying the confidential information found in the electronic medical records while maintaining the anonymity of the research subjects. However, as our inspection supervisions—as stated earlier—do not substitute those of the committees, verification procedures were developed, or recommendations were made in the supervision minutes for specific aspects to be reviewed by the ethics committee.
Internet access was noted to be a persistent problem, and it was related to the fact that our study was conducted in a country with lower bandwidth than other Latin American countries and that connectivity is deficient in some regions of the country. These inconveniences were addressed using other methods, such as WhatsApp, to share the requested documents.
Despite the challenges, the virtual inspection implementation process included a validation period that had already been published [3], which systematized a procedure that produced instructions that were recognized as simple or adequate. As a result, despite the difficulties, the assessment of the supervision process was found to be positive by both the main researchers and the study coordinators. This could also be related to the willingness of the main researchers and study coordinators to undergo supervision as they believed that it promoted compliance with GCP and bioethical principles. In this context, to ensure quality in the development of a CT, it is necessary that the main researchers and their team comply with the GCP. GCP are considered as quality and control tool, and they include surveillance visits, audits, and inspections, among other items [11].
Our results show the feasibility of virtual inspections to CTs carried out in EsSalud. Despite the fact that the physical distancing provisions ended in our country, the good perception of quality, in addition to the possibility of saving resources in inspections outside the country's capital, unlike face-to-face inspections that were mostly in the capital, encourage to continue with virtual inspections. This experience could be welcomed by other countries in the region where the pandemic posed challenges for the inspection of CTs.
In Guatemala, the pandemic forced the office responsible for CTs to switch to a virtual format for communication between the regulatory entity and the private research companies [Contract Research Organization or Site Management Organization (SMO)]. This essentially affected the procedures for the delivery of documentation for the authorization of CTs, i.e., moving from a face-to-face format [11] to using e-mails, without the decision being supported by a documented regulatory directive. Regarding the current web page, the phrase “Submit to the Pharmacovigilance and Clinical Trials office...” [12] appeared, making a reference to the documentation for CT authorization. The use of video recordings was also implemented for the approval of research sites, without the instruction being supported by a regulation. These two tools have replaced the procedures that previously had to be performed using printed documentation and on-site visits to the study sites. The lack of a normative directive in this process affects the governance of the research system.
Regarding the inspection of CTs at research sites during the pandemic period, the regulatory body in Guatemala did not address the issue, and only a written report was required every six months for clinical trial monitoring and obtaining authorization to continue the recruitment of research subjects. The pandemic did not affect the volume of CT research conducted between 2020 and 2021, with the approval of 20 protocols each year; of these, 4 CTs related to COVID-19 were approved in 2021 [13]. Notably, in Guatemala, a new CT regulation [14]. was recently approved and published at the end of 2021; however, this does not mean that the virtual format will be incorporated into the regulations on the inspection of CTs. Proposing procedures adapted to the new circumstances with the use of technology is an innovative challenge for the regulatory and ethical aspects.
In Guatemala, the Ministry of Public Health, through the office responsible for CTs, generated virtual measures to simplify the processes (it did not represent a virtual platform). However, during the pandemic in Guatemala, two versions of the standard governing CTs were adopted, and issues, such as virtuality, were not included, despite the pandemic disrupting face-to-face processes. At least one ethics committee, the most sought-after in Guatemala, adopted e-mail communication because of the pandemic. The purpose of the virtual communication was to exchange documents and not necessarily to set up an inspection mechanism, which has been suspended.
In Argentina, the experience of developing and to inspect CT in the context of the pandemic differed among research centers, including the modification of standard operating procedures to adapt to the pandemic and the accelerated evaluation of protocols related to COVID-19 [15]. Similarly, the National Administration of Medicines, Food and Medical Technology (ANMAT)—as the regulatory body in Argentina—established measures and recommendations for the development of clinical pharmacology studies (CPS); as a part of these recommendations, sponsors were required to implement risk mitigation plans to avoid infections, dissemination of COVID-19, and saturation of the health system. Measures have also been suggested to examine study participants through telemedicine and home visits for the performance of procedures and delivery of the investigational drug [16].
Moreover, in Argentina, the evaluations of CPS were conducted virtually, through a platform in which smooth communication was established via institutional e-mail and virtual meetings between the sponsor and the CT Department of the regulatory body. Regarding follow-up of the approved COVID-19 CTs, follow-ups were carried out via e-mails, and the sponsors were responsible for sending the status of the studies for monitoring and supervision. Regarding progress reports, the frequency was higher in CTs with COVID-19 vaccines because they required more follow-ups and monitoring [17]. Finally, the CT Inspection Service of the Argentine regulatory agency ANMAT developed a new tool for the Procedure for Remote Inspections of GCP [17].
Limitations
Our study had some limitations. First, the supervision of CTs was performed in the EsSalud, which is not necessarily applicable to those developed at other institutions that do not have an institution, such as IETSI, for the inspection of CTs. Second, the assessment of perceptions did not use a validated instrument. However, an instrument with Likert-type questions was used, which helped provide an idea of what we were interested in knowing. Third, although it was an anonymous evaluation, our institution was the one to inquire about how virtual inspection was perceived; thus, it was possible that the favorable perception was overestimated. However, to the best of our knowledge, this was the first study to evaluate the lessons learned in the VS of a CT implementation process, and it allows us to collect information for improving the inspection process at our institution.
Conclusion
Discrepancies in the records and failure to submit some of the requested documents were the main observations during the virtual inspection. These problems were resolved using other methods, such as WhatsApp, to share the requested documents. Moreover, most interviewees considered the material used to be adequate and gave an overall good rating to the virtual inspection process. Despite the difficulties, the good perception of the virtual inspections of the CTs, as a regulatory entity, we will continue with this modality, which could be replicated by other countries in the region.
References
“Ministry of Health, Clinical Trials Regulations,” Lima, MINSA, 2018, https://repositorio.ins.gob.pe/xmlui/bitstream/handle/INS/1113/ENSAYOS%20CL%c3%8dNICOS%202018.pdf?sequence=1&isAllowed=y.
Martinez R. Clinical trial outcomes: what matters to patients. JACC Heart Fail. 2019;7:272–3.
Herrera-Añazco P, Soto-Ordoñez S, Estrada-Martínez M. Procedure for the creation of a virtual tool for the administrative supervision of clinical trials conducted in the peruvian social security. Med Corps Mag Hosp Nacional Almanzor Aguinaga Asenjo. 2021;14:244–5.
García-Mostajo JA, et al. Supervision of clinical trials in peruvian social health insurance hospitals: administrative and regulatory approach. Revista Peruana de Medicina Experimental y Salud Publica. 2019;36:687–91.
Lope PC, et al. The Regulation of Clinical Trials for COVID-19 in Peru. Revista Peruana de Medicina Experimental y Salud Publica. 2021;38:171–7.
“Council for International Organizations of Medical Sciences (CIOMS) in collaboration with the World Health Organization (WHO),” International Ethical Guidelines for Health-Related Research Involving Human Subjects, 2016, https://cioms.ch/wp-content/uploads/2017/12/CIOMS-EthicalGuideline_SP_INTERIOR-FINAL.pdf
Castro M, et al. Ethical aspects of the most commonly used designs in clinical research. J Med Sci Health (Chile). 2019;5:183–93.
Herrera-Añazco, P., et al., “Situational Diagnosis of the Institutional Research Ethics Committees of the Social Security in Peru,” Revista del Cuerpo Médico Hospital Nacional Almanzor Aguinaga Asenjo March 31, 2022, https://cmhnaaa.org.pe/ojs/index.php/rcmhnaaa/article/view/1057.
RJ_096–2020-J-OPE-INS, Forming the National Transitory Committee on Research Ethics for the Evaluation and Ethical Supervision of Clinical Trials of COVID-19 Disease, https://cdn.www.gob.pe/uploads/document/file/1049675/RJ_096-2020-J-OPE-INS20200727-24078-s7qgfw.pdf.
“International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use,” Int Dig Health Legis. 1997;48:231–4.
“Pan American Health Organization (PAHO),” 2019, Good Clinical Practices: document of the Americas, www.paho.org/hq/index.php.
“Department of Regulation and Control of Pharmaceutical and Related Products,” Ministry of Public Health and Social Assistance. Government of Guatemala, Clinical Trial Forms, https://medicamentos.mspas.gob.gt/index.php/formularios/formensayos.
“MSPAS,” Clinical Trial Protocols, 2022, https://medicamentos.mspas.gob.gt/index.php/consultas/protocolo.
“MSPAS.” Regulations for the Regulation of Human Clinical Trials, 2022, https://medicamentos.mspas.gob.gt/index.php/legislacion-vigente/acuerdos?download=336%3Aacuerdo-ministerial-206-2021.
Ana, P., et al., “The State of the Research Ethics Evaluation System in Argentina and Its Adaptation to the COVID-19 Pandemic,” Revista Argentina de Salud Pública, 2020, http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1853-810X2020000300013&lng=es&nrm=iso.
“National Administration of Medicines, Food and Medical Technology (ANMAT),” Actions and Recommendations in Clinical Pharmacology Studies During the COVID-19 Pandemic, 2020, https://www.argentina.gob.ar/noticias/medidas-y-recomendaciones-en-los-estudios-de-farmacologia-clinica-durante-la-pandemia-covid.
Laura, T., et al., “Regulatory approach and analysis of the evaluation of clinical pharmacology studies by the National Administration of Medicines, Food and Medical Technology (ANMAT), during the period of the COVID-19 pandemic.” ANMAT Scientific Journal, 2021, https://www.argentina.gob.ar/sites/default/files/articulo_enfoque_regulatorio._-_anmat-_aprobado_para_publicar_25-11-21_-_revista_cientifica_anmat.pdf.
Funding
This was a self-financed study.
Author information
Authors and Affiliations
Contributions
PHA, DUP, and SSO: designed the study. DUP, SSO, AMCT, FT, and LMLD: performed the analyses. All authors interpreted the results, drafted the manuscript, and critically reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herrera-Añazco, P., Urrunaga-Pastor, D., Soto-Ordoñez, S. et al. Results and Feasibility of the Virtual Inspection of Clinical Trials During Pandemic of COVID-19 in Peru. Ther Innov Regul Sci 57, 678–688 (2023). https://doi.org/10.1007/s43441-023-00503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-023-00503-7