Abstract
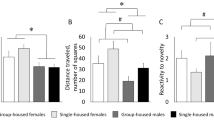
The aim of the present study was to assess the influence of experimential factors on the vulnerability of rats to develop amphetamine (AMPH)- and stressor-induced behavioral sensitization. Young male Wistar rats with previous social experience were isolated from their peers for 2 weeks. 1) The effect of this short-lasting social deprivation were: a) a reduced tendency to explore a fearful environment; b) a prolonged exploratory activity in response to a novel but little fearful environment; and c) a dose-dependent increase in the psychomotor stimulation induced by systemic AMPH injection. 2) After repeated AMPH injections (injection every other day for 10 days), isolated rats exhibited behavioral sensitization at lower doses (0.5 and 0.75 mg/kg) than those required for group-housed rats (1 mg/kg). 3) After being submitted to a repeated stressor (3, 7 or 14 footshock sessions, with 2 days between sessions), the isolated rats exhibited a greater increase in the behavioral responsivity to a subsequent AMPH challenge (1 mg/kg) than did the group-housed rats regardless of the number of stress sessions. In conclusion, these results suggest that experiential factors such as privation of contact with peers (social isolation) may make rats more vulnerable to the long-term repercussions of chronic environmental and pharmacological challenges.
Similar content being viewed by others
References
Ahmed SH, Spampinato U, Stinus L, Le Moal M, Cador M (1993) social deprivation increases the vulnerability of male Wistar rats to develop behavioral sensitization tod-amphetamine. Behav Pharmacol 4:450
Altenor A, Kay E (1980) The effects of postweaning rearing conditions on the response to stressful tasks in the rat. Physiol Psychol 8:88–92
Anisman H, Sklar LS (1981) Social housing conditions influence escape deficits produced by uncontrollable stress: assessment of the contribution of norepinephrine. Behav Neural Biol 32:406–427
Antelman SM (1988) Stressor-induced sensitization to subsequent stress: implications for the development and treatment of clinical disorders. In: Kalivas PW, Barnes CD (eds) Sensitization in the nervous system. Telford Press, Caldwell, NJ, pp 228–254
Antelman SM, Chiodo LA (1983) Amphetamine as a stressor. In: Creese I (ed) Stimulants: neurochemical, behavioral and clinical perspectives. Raven Press, New York, pp 269–299
Antelman SM, Eichler AJ, Black CA, Kocan D (1980) Interchang-eability of stress and amphetamine in sensitization. Science 207:329–331
Bickerdike MJ, Wright IK, Marsden CA (1993) social isolation attenuates rat forebrain 5-HT release induced by KCl stimulation and exposure to a novel environment. Behav Pharmacol 4:231–236
Blanc G, Hervé D, Simon H, Lisoprawski A, Glowinski J, Tassin JP (1980) Response to stress of mesocortical-frontal dopaminergic neurons in rats after long-term isolation. Nature 284:265–267
Boyle AE, Gill K, Smith BR, Amit Z (1991) Differential effects of an early housing manipulation on cocaine-induced activity and self-administration in laboratory rats. Pharmacol Biochem Behav 39:269–274
Camp DM, Robinson TE (1988) Susceptibility to sensitization. I. Sex differences in the enduring effects of chronicd-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res 30:55–68
Chitkara B, Durcan MJ, Campbell IC (1984) Apomorphine-induced stereotypy: function of age and rearing environment. Pharmacol Biochem Behav 21:671–673
Cole BJ, Cador M, Stinus L, Rivier J, Vale W, Koob GF, Le Moal M (1990) Central administration of a CRF antagonist blocks the development of stress-induced behavioral sensitization. Brain Res 512:343–346
Dalrymple-Alford JC, Benton D (1981) The effect of social isolation of the rat on open-field activity and emergence. Behav Proc 6:283–290
Fujiwara Y, Kazahaya Y, Nakashima M, Sato M, Otzuki S (1987) Behavioral sensitization to metamphetamine in the rat: an ontogenic study. Psychopharmacology 91:316–319
Gentsch C, Lichtsteiner M, Feer H (1981) Locomotor activity, defecation score and corticosterone levels during an openfield exposure: a comparison among individually and group-housed rats, and genetically selected rat lines. Physiol Behav 27:183–186
Gentsch C, Lichtsteiner M, Frischknecht HR, Feer H, Siegfried B (1988) Isolation-induced locomotor hyperactivity and hypoalgesia in rats are prevented by handling and reversed by resocialisation. Physiol Behav 43:13–16
Geyer M, Wilkinson LS, Humby T, Robbins TW (1993) Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry 34:361–372
Giralt M, Armario A (1989) Individual housing does not influence the adaptation of the pituitary-adrenal axis and other physiological variables to chronic stress in adult male rats. Physiol Behav 45:477–481
Haney M, Castanon N, Cador M, Le Moal M, Mormède P (1994) Cocaine sensitization in Roman High and Low Avoidance rats is modulated by sex and gonadal hormone status. Brain Res 695:179–185
Heritch AJ, Henderson K, Westfall TC (1990) Effects of social isolation on brain catecholamines and forced swimming in rats: prevention by antidepressant treatment. J Psychiatr Res 24:251–258
Herman JP, Stinus L, Le Moal M (1984) Repeated stress increases locomotor response to amphetamine. Psychopharmacology 84:431–435
Holson RR, Scallet AC, Ali SF, Turner BB (1991) “Isolation stress” revisited: isolation-rearing effects depend on animal care methods Physiol Behav 49:1107–1118
Hooks MS, Jones GH, Neill DB, Justice JB (1991a) Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav 38:467–470
Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB (1991b) Individual differences in amphetamine sensitization: dose-dependent effects. Pharmacol Biochem Behav 41:203–210
Jankowska E, Pucilowski O, Kostowski W (1991) Chronic oral treatment with diltiazem or verapamil decreases isolation-induced activity impairment in elevated plus maze. Behav Brain Res 43:155–158
Jones GH, Robbins TW, Marsden CA (1989) Isolation-rearing retards the acquisition of schedule-induced polydipsia in rats. Physiol Behav 45:71–78
Jones GH, Marsden CA, Robbins TW (1990) Increased sensitivity to amphetamine and reward-related stimuli following social isolation in rats: possible disruption of dopamine-dependent mechanisms in the nucleus accumbens. Psychopharmacology 102:364–372
Jones GH, Hernandez TD, Kendall DA, Marsden CA, Robbins TW (1992) Dopaminergic and serotonergic function following isolation-rearing in rats: study of behavioural responses and postmortem and in vivo neurochemistry. Pharmacol Biochem Behav 43:17–35
Kalivas PW (1993) Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev 18:75–113
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev 16:223–244
Kelley AE, Cador M (1988) Behavioral evidence for differential neuropeptide modulation of the mesolimbic dopamine system. Ann NY Acad Sci 537:415–434
Kolta MG, Scalzo FM, Ali SF, Holson RR (1990) Ontogeny of the enhanced behavioral response to amphetamine in amphetamine-pretreated rats. Psychopharmacology 100:377–382
Leith NJ, Kuczenski R (1982) Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology 76:310–315
Maisonnette S, Morato S, Brandao ML (1993) Role of resocialisation and 5-HT1A receptor activation on the anxiogenic effects induced by isolation in the elevated plus-maze test. Physiol Behav 54:753–758
Mayer LA, Parker LA (1993) Rewarding and aversive properties of IP and SC cocaine: assessment by place and taste conditioning. Psychopharmacology 112:189–194
McLennan AJ, Maier SF (1983) Coping and stress-induced potentiation of stimulant stereotypies in the rat. Science 219:1091–1093
Motta V, Maisonnette S, Morato S, Castrechini P, Brandao ML (1992) Effects of blockade of 5-HT2 receptors and activation of 5-HT1A receptors on the exploratory activity of rats in the elevated plus-maze. Psychopharmacology 107:135–139
Nomikos GG, Spyraki C (1988) Cocaine-induced place conditioning: importance of route of administration and others variables. Psychopharmacology 94:119–125
Oehler J, Jähkel M, Schmidt J (1987) Neuronal transmitter sensitivity after social isolation in rats. Physiol Behav 41:187–191
Phillips GD, Robbins TW, Whitelaw RB, Howes SR, Everitt BJ (1993) Isolation-rearing increases locomotor activity in response to an injection of cocaine or to a novel environment, but decreases the intravenous self-administration of cocaine or the intra-accumbens self-administration ofd-amphetamine. Behav Pharmacol 4:458
Piazza PV, Deminière JM, Le Moal M, Simon H (1990) Stress- and pharmacologically-induced behavioral sensitization increases the vulnerability to acquisition of amphetamine self-administration. Brain Res 514:22–26
Post RM (1980) Intermittent versus continuous stimulation: effect of time interval on the development of sensitization of tolerance. Life Sci 26:1275–1282
Post RM (1992) Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 149:999–1010
Post RM, Contel NR, Gold PW (1982) Impaired behavioral sensitization to cocaine in vasopressin deficient rats. Life Sci 31:2745–2950
Robinson TE (1984) Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology 84:466–475
Robinson TE (1988) Stimulants drugs and stress: factors influencing individual differences in the susceptibility to sensitization. In: Kalivas PW, Barnes CD (eds) Sensitization in the nervous system. Telford Press, Caldwell NJ, pp 145–174
Robinson TE, Angus AL, Becker JB (1985) Sensitization to stress: the enduring effects of prior stress on amphetamine-induced rotational behavior. Life Sci 37:1039–1042
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev 11:157–198
Robinson TE, Becker JB, Presty SK (1982) Long term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res 253:231–241
Sahakian BJ, Robbins TW (1977) Isolation-rearing enhances tail-pinch-induced oral behaviour in rats. Physiol Behav 18:53–58
Sahakian BJ, Robbins TW, Morgan MJ, Iversen SD (1975) The effects of psychomotor stimulants on stereotypy and locomotor activity in socially-deprived and control rats. Brain Res 84:195–205
Sahakian BJ, Robbins TW, Iversen SD (1977) The effects of isolation-rearing on exploration in the rat. Anim Learn Behav 5:193–198
Schenk S, Robinson B, Amit Z (1988) Housing conditions fail to affect the intravenous self-administration of amphetamine. Pharmacol Biochem Behav 31:59–62
Segal DS, Schuckit MA (1983) Animal models of stimulant-induced psychosis. In: Creese I (ed) Stimulants: neurochemical, behavioral and clinical perspectives. Raven Press, New York, pp 131–167
Segal DS, Kuczenski R (1987) Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. Pharmacol Exp Ther 242:917–926
Segal DS, Mandell AJ (1974) Long-term administration of amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 2:249–255
Sorg BA, Kalivas PW (1991) Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res 559:29–36
Stewart J, Vezina P (1987) Environment-specific enhancement of the hyperactivity induced by systemic or intra-VTA morphine injections in rats preexposed to amphetamine. Psychobiology 15:144–153
Stinus L, Cador M, Le Moal M (1992) Interaction between endogenous opioids and dopamine within the nucleus accumbens. Ann NY Acad Sci 654:254–273
Vasar E, Peuranen E, Harro J, Lang A, Oreland L, Männisto PT (1993) social isolation of rats increases the density of cholecystokinin receptors in the frontal cortex and abolishes the anti-exploratory effect of caerulein. Arch Pharmacol 348:96–101
Viken RJ, Moore DL, Knutson JF, Fordyce DJ (1982) The effects of social isolation and handling on reactivity to footshock and tactile dorsal stimulation. Anim Learn Behav 10:135–140
Wade SE, Maier SF (1986) Effects of individual housing and stressor exposure upon the acquisition of watermaze escape. Learn Motiv 17:287–310
Weinstock M, Speiser Z, Ashkenzi R (1978) Changes in brain catecholamine turnover and receptor sensitivity induced by social deprivation in rats. Psychopharmacology 56:205–209
Wright IK, Upton N, Marsden CA (1991) Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physio Behav 50:1129–1132
yehuda R, Antelman SM (1993) Criteria for rationally evaluating animal models of posttraumatic stress disorder. Biol Psychiatry 33:479–486
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ahmed, S.H., Stinus, L., Le Moal, M. et al. Social deprivation enhances the vulnerability of male Wistar rats to stressor- and amphetamine-induced behavioral sensitization. Psychopharmacology 117, 116–124 (1995). https://doi.org/10.1007/BF02245106
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02245106