Abstract
Introduction
Early use of corticosteroids in patients affected by pandemic (H1N1)v influenza A infection, although relatively common, remains controversial.
Methods
Prospective, observational, multicenter study from 23 June 2009 through 11 February 2010, reported in the European Society of Intensive Care Medicine (ESICM) H1N1 registry.
Results
Two hundred twenty patients admitted to an intensive care unit (ICU) with completed outcome data were analyzed. Invasive mechanical ventilation was used in 155 (70.5%). Sixty-seven (30.5%) of the patients died in ICU and 75 (34.1%) whilst in hospital. One hundred twenty-six (57.3%) patients received corticosteroid therapy on admission to ICU. Patients who received corticosteroids were significantly older and were more likely to have coexisting asthma, chronic obstructive pulmonary disease (COPD), and chronic steroid use. These patients receiving corticosteroids had increased likelihood of developing hospital-acquired pneumonia (HAP) [26.2% versus 13.8%, p < 0.05; odds ratio (OR) 2.2, confidence interval (CI) 1.1–4.5]. Patients who received corticosteroids had significantly higher ICU mortality than patients who did not (46.0% versus 18.1%, p < 0.01; OR 3.8, CI 2.1–7.2). Cox regression analysis adjusted for severity and potential confounding factors identified that early use of corticosteroids was not significantly associated with mortality [hazard ratio (HR) 1.3, 95% CI 0.7–2.4, p = 0.4] but was still associated with an increased rate of HAP (OR 2.2, 95% CI 1.0–4.8, p < 0.05). When only patients developing acute respiratory distress syndrome (ARDS) were analyzed, similar results were observed.
Conclusions
Early use of corticosteroids in patients affected by pandemic (H1N1)v influenza A infection did not result in better outcomes and was associated with increased risk of superinfections.
Similar content being viewed by others
Introduction
Pandemic (H1N1)v influenza A infection was first described in Mexico in April 2009, and on 11 June 2009 the World Health Organization (WHO) declared the new flu as the first pandemia of the 21 century, by which time mortality rates were being reported to be as high as 38% [1] with similar rates being reported in all continents: Spain (25%) [1], Canada (17.3%) [2], Australia and New Zealand (14.3%) [3], and Argentina (46%) [4].
The efficacy of systemic corticosteroids has been extensively studied in acute respiratory distress syndrome (ARDS). While they clearly have a role in situations where ARDS has been precipitated by a corticosteroid-responsive process (e.g., acute eosinophilic pneumonia), the value of corticosteroid therapy in most other cases remains uncertain [5]. In the 1970s and early 1980s, empirical corticosteroids were widely used to treat ARDS; however, corticosteroid therapy in this setting subsequently became less frequent after several studies found that they had no benefit and may actually cause harm [6, 7]. Since then, several meta-analyses and reviews have been published offering conflicting perspectives regarding corticosteroid treatment for ARDS [8–11].
The most common pulmonary presentation of patients affected by pandemic (H1N1)v influenza A infection is rapidly progressive viral pneumonia with bilateral alveolar infiltrates on chest radiography, and ARDS [12]. The presentation of ARDS with severe refractory hypoxemia has been particularly common in patients with this disease process and might be linked to an abnormal immune response [13]. Several published reports of pandemic (H1N1)v influenza A infection [2, 14] have reported use of empirical corticosteroid therapy in more than half of these patients, both as primary therapy and as rescue therapy for patients with severe ARDS. Recent guidelines for management of human infection with pandemic (H1N1)v influenza A infection recommend that corticosteroid therapy should not be used routinely, although low doses may be considered for patients in septic shock who require vasopressors and have suspected adrenal insufficiency [15, 16]. Data supporting this guidance, however, remain scarce and controversial [17]. A single prospective interventional study with only 13 patients by Quispe-Laime et al. [18] demonstrated that a prolonged low to moderate dose of corticosteroid treatment was associated with significant improvement in lung injury and multiple organ dysfunction scores and reduced hospital mortality rate.
The main objective of this study is therefore to assess the effect on survival of early corticosteroid therapy compared with those who did not receive corticosteroids or received them subsequently as rescue therapy, in a cohort of patients hospitalized with severe presentation of pandemic (H1N1)v influenza A infection in the ICU.
Materials and methods
Data for this study were obtained from a voluntary registry instituted by the European Society of Intensive Care Medicine (ESICM). The registry contains data from patients admitted to the ICU with confirmed, probable or suspected pandemic (H1N1)v influenza A infection. All reports notified before 11 February 2010 were eligible for inclusion. Ethical approval was sought and obtained where necessary prior to any patients being entered into the registry. All patients enrolled were recorded into the registry in an anonymous format. The need for informed consent was waived due to the observational nature of the study and the fact that this activity was an emergency public health response.
The inclusion criteria for this study consisted of: fever (>38°C); acute illness; respiratory symptoms consistent with cough, sore throat, myalgia or influenza-like illness; and acute respiratory failure requiring ICU admission with confirmed, probable or suspected pandemic (H1N1)v influenza A infection, according to case definitions developed by the World Health Organization (WHO) [19, 20]. A “confirmed case” was defined as an acute respiratory illness with laboratory-confirmed pandemic (H1N1)v influenza A virus infection with real-time reverse-transcription polymerase chain reaction (RT-PCR) or viral culture [20]. All tests and procedures were ordered by attending physicians.
The definitions of community-acquired pneumonia and hospital-acquired pneumonia were based on 2007 American Thoracic Society and Infectious Disease Society of America guidelines [21]. Primary viral pneumonia was defined in patients presenting during the acute phase of influenza virus illness with ARDS and unequivocal alveolar opacification involving two or more lobes with negative respiratory and blood bacterial cultures. Secondary bacterial pneumonia was considered in patients with confirmation of influenza virus infection who showed recurrence of fever, increase in cough, and production of purulent sputum with in addition positive respiratory pathogens or blood cultures [22]. Microbiological confirmation of HAP was based on standardized procedures at each investigator site. Acute renal failure was defined as need for renal replacement therapy following the International Consensus Conference [23]. Obese patients were defined as those with body mass index (BMI) over 30 kg/m2 [24]. ICU admission criteria and treatment decisions for all patients, including determination of need for intubation and type of antibiotic and antiviral therapy administered, were made by the attending physician. The following information was also recorded: demographic data, comorbidities, time of illness onset and hospital admission, time to first dose of antiviral therapy, microbiologic findings, and chest radiographic findings at ICU admission. Intubation and mechanical ventilation requirements, adverse events during ICU stay (e.g., need for vasopressor drugs, or renal replacement techniques), and laboratory findings at ICU admission were also recorded. To determine illness severity, the Simplified Acute Physiology Score (SAPS3) [25, 26] and the Acute Physiology and Chronic Health Evaluation (APACHE) II score [27] were determined in all patients within 24 h of ICU admission. In addition, organ failure was assessed using the Sequential Organ Failure Assessment (SOFA) scoring system [28].
Systemic corticosteroid use was considered when dosages equivalent to >24 mg/day methylprednisone or >30 mg/day prednisone were given at ICU admission. Patients who received corticosteroid therapy on ICU admission were compared with those who did not receive corticosteroid therapy or who received them subsequently as rescue therapy for unfavorable clinical progression.
Statistical analysis
Discrete variables are described as counts (%) and continuous variables as mean with standard deviation (SD) or median with 25th to 75th interquartile range (IQR), as appropriate. Unless otherwise stated, all statistical tests were two sided and p < 0.05 was considered significant. Differences in categorical variables were calculated using the two-sided likelihood ratio, chi-square test or Fisher’s exact test, and the Mann–Whitney U test or Kruskal–Wallis test was used for continuous variables, when appropriate. Cox proportional-hazards regression analysis was used to assess the impact of independent variables on ICU mortality across time. Variables significantly associated with mortality on univariate analysis were entered into the model. To avoid spurious associations, variables entered into the regression models were those with a relationship on univariate analysis (p ≤ 0.05) or a plausible relationship with the dependent variable. Results are presented as hazard ratio (HR) and 95% confidence interval (CI). Potential explanatory variables were checked for collinearity prior to inclusion in the regression models using tolerance and variance inflation factor. Data analysis was performed using SPSS 13.0 (SPSS, Chicago, IL, USA) for Windows.
Results
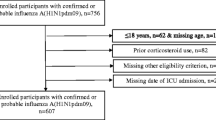
Two hundred twenty patients with completed outcomes from the ESICM H1N1 registry were analyzed in this study. All patients had suspected, probable or confirmed pandemic (H1N1)v influenza A infection and were being cared for in an ICU. One hundred ninety-four were confirmed (88.2%), 2 were probable (0.9%), and 24 patients were suspected (10.9%) for pandemic (H1N1)v influenza A virus. Of these, 113 patients were male (51.4%) with median age of 43 (IQR 32–55) years, and 188 (85.5%) were under 60 years of age. The mean SAPS3 score was 53.0 ± 16.2 and the mean SOFA score was 8.2 ± 4.2 on admission. Mechanical ventilation was used in 171 (77.7%) of the patients, 155 (70.5%) with invasive modes and 65 (29.5%) noninvasively; 49 (75.3%) of the patients having noninvasive modes of ventilation subsequently required invasive ventilation. All patients received antiviral therapy. Oseltamivir administration delay after illness onset did not differ between early corticosteroid uses. ARDS was present in 74.3% patients. Comorbidities were present in 107 (48.6%) patients. Obesity (n = 67, 30.5%), asthma (n = 24, 10.9%), and chronic obstructive pulmonary disease (COPD, n = 23 10.5%) were the main comorbidities reported.
One hundred twenty-six (57.3%) patients received early corticosteroid therapy at ICU admission. Patients surviving the ICU stay and receiving corticosteroids early on ICU admission had mean duration of corticosteroid therapy of 10.3 ± 11.7 days. ICU length of stay in survivors did not differ in patients who received early corticosteroids compared with those who did not (12.9 ± 14.0 versus 10.8 ± 9.8 days, p = 0.29). Patients who received early corticosteroid therapy were significantly older (46.2 ± 14.9 versus 39.4 ± 17.1 years, p < 0.001) and had asthma [19 (15.1%) versus 5 (5.3%), p < 0.001], COPD [19 (15.1%) versus 4 (4.3%), p < 0.01], and chronic steroid use [24 (19%) versus 4 (4.3%), p < 0.01] more frequently than patients who did not. Patients who received early corticosteroid therapy were sicker than those who did not receive them according to SAPS3 data (55.9 ± 16.8 versus 49.0 ± 14.5, p = 0.001). No differences were found between patients who were or were not treated with early corticosteroid therapy regarding prevalence of ARDS (70.4% versus 77.3%, p 0.3). Mechanical ventilation was based on lung protective strategies. For the entire cohort, tidal volume was 5.7 (IQR 4.7–6.5) ml/kg ideal body weight (IBW). We did not find any differences between tidal volume in patients who received early corticosteroid therapy compared with those who did not [5.6 (IQR 4.7–6.3) versus 5.7 (IQR 4.8–7.5) ml/kg IBW, p = 0.2]. Additional demographic data and clinical characteristics of patients with pandemic (H1N1)v influenza A with and without early corticosteroid therapy are presented in Table 1.
Hospital-acquired pneumonia was clinically suspected in 79 patients (35.9%), with microbiological documentation in 46 patients (20.9%) patients. Patients who received early corticosteroid therapy had HAP more frequently than patients who did not [26.2% versus 13.8%, p < 0.05; odds ratio (OR) 2.2, CI 1.1–4.5]. Since the severity of illness of patients who received early corticosteroid therapy was higher, multivariate regression analysis adjusting for severity was performed and confirmed the higher incidence of HAP in patients who received early corticosteroid therapy [OR = 2.2 95%, confidence interval (CI) 1.0–4.8; p < 0.05]. Pseudomonas aeruginosa (n = 13, 28.3%) was identified as the most prevalent pathogen, followed by Acinetobacter baumannii (n = 7, 15.2%) and Streptococcus pneumoniae (n = 5, 10.9%) (Table 2).
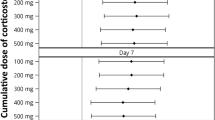
In total, 67 patients died on the ICU (30.5%) and 75 (34.1%) whilst in hospital. Nonsurvivors presented with significantly higher SAPS3 score at admission (61.9 ± 18.5 versus 47.7 ± 11.9, p < 0.01) and higher SOFA score (10.1 ± 4.2 versus 6.7 ± 3.7, p < 0.01) when compared with survivors. The characteristics of the patients who died are shown in Table 3. Patients who received early corticosteroid therapy on ICU admission had significantly higher ICU mortality than those who did not (46.0% versus 18.1%; OR 3.8, CI 2.1–7.2; p < 0.01). This association with increased mortality was not present when mortality data were adjusted for increased severity of illness (SAPS3) and other known confounding variables (age, COPD, asthma, and chronic steroid use) [hazard ratio (HR) 1.3 95%, CI 0.7–2.4; p = 0.4] (Fig. 1). Similar findings were found when repeating the analysis for only the cohort of patients who presented with ARDS (HR 1.1, 95% CI 0.5–2.3; p = 0.7).
Discussion
This analysis of a large, cohort, prospective, multicenter research study suggests that prompt use of corticosteroid therapy on ICU admission does not result in a reduction of mortality for critically ill patients admitted with pandemic (H1N1)v influenza A infection. Furthermore, there is also not a beneficial effect of early corticosteroid therapy when given to the more severe end of the spectrum of patients requiring invasive mechanical ventilation for ARDS. Another important finding of this study was that patients receiving early corticosteroid therapy had increased likelihood of developing superadded bacterial infection.
Endogenous glucocorticoids as end-effectors play a role in inhibiting inflammation [29] but are not always effective in suppressing the “cytokine storm” driven by systemic inflammation, even though cortisol levels have been correlated with grades of illness severity and mortality [30]. With the concept of critical-illness-related corticosteroid insufficiency (CIRCI) [31] and the results of clinical trials showing respiratory immune and hemodynamic benefits, corticosteroid therapy has re-emerged as a promising adjunct for treatment of severe sepsis.
Severe bacterial pneumonia is associated with relative corticosteroid insufficiency as well as a plethora of other pulmonary and systemic effects [32]. This inflammatory cascade can be partially blocked by administration of systemic corticosteroid therapy [33]. The more severe the presentation, the worse the inflammatory crisis, therefore previous authors have suggested that steroid therapy should be more effective in more severely ill patients [34–36]. This is not what was shown in the present study. Recent guidelines for management of community-acquired pneumonia suggest the benefit of systemic corticosteroid therapy for patients with severe presentation [37]. This has been shown in one small randomized controlled study with hydrocortisone treatment, terminated prematurely due to 0% mortality in the intervention arm and a significant reduction in length of hospital stay [38]. More recently, Snijders et al. [39] conducted a randomized controlled trial in 213 hospitalized patients with CAP. These patients were randomized to receive either 40 mg prednisolone for 7 days or placebo added to antibiotic therapy. This study did not show any differences in clinical outcomes in either the overall population or those with severe pneumonia. Additionally, late clinical failure (>72 h after hospital admission) was more common in the prednisolone group than in the placebo group.
Data supporting use of corticosteroid therapy in patients affected by primary viral pneumonia are limited at the present time, with some reports extrapolating from the 2002–2003 severe acute respiratory syndrome coronavirus (SARS-CoV) outbreak [40–42] to the current pandemic. The innate antiviral host response is based on early elevated expression of CXCL10, CCL2, and CCL4 in SARS-CoV and human respiratory syncytial virus (hRSV)-infected patients [43–45]. Use of corticosteroid therapy is a double-edged sword. Li et al. [46] reported that high doses of corticosteroids decrease immunity by reducing CD4, CD8, and CD3 levels in patients with SARS and result in an increase in secondary infections; moreover, Tsang et al. [47] found an increase in 30-day mortality in the same subset of patients. Nevertheless, the exact mechanism of corticosteroid therapy needs to be further elucidated due to the fact that there is no evidence of benefit in SARS in the early phase when SARS-CoV replication is still ongoing. Lee et al. [48] found that SARS-CoV load was significantly higher in the second and third week of illness in patients who received initial corticosteroid therapy.
Recent results from the CORTICUS study [49] do not support routine use of corticosteroid therapy in patients with septic shock, because they showed only a beneficial effect of stress doses of corticosteroids in decreasing time to shock reversal [50] but not on 28-day mortality, an effect at least in part explained by an increased risk of superinfection. Use of corticosteroid therapy also exerts a decisive influence on the immune function of macrophages and granulocytes, the main cell host defenses against bacteria [51]. In the present study there was significant incidence of nosocomial infections that resulted in twofold higher incidence of hospital-acquired pneumonia in patients who received corticosteroid therapy. Incidence of HAP due to Pseudomonas aeruginosa was 92.3% and due to Acinetobacter baumannii was 71.4% in the corticosteroid group. Additionally, one of the most intriguing observations was that four patients developed ventilator-associated pneumonia (VAP) due to Aspergillus spp., three of whom were receiving corticosteroid therapy.
The present study has several potential limitations that should be addressed. First is that only patients treated with early corticosteroid therapy on ICU admission were considered in the treatment group. The control group comprised patients who did not receive early corticosteroid therapy and those who received them subsequently as rescue therapy. Use of corticosteroid therapy after ICU admission was not considered in the treatment group, since this subgroup of patients would be considered as receiving rescue therapy due to unfavorable clinical progression. No data were available to subanalyze the role of rescue therapy; nevertheless, the multivariate analysis was adjusted for severity as well as other confounding factors to avoid a potential bias that might invalidate our final conclusions. Secondly, this is an observational, noninterventional study, in which the participating ICUs from 23 countries in the world were self-selected. Prescription of corticosteroids was chosen in accordance with local protocols. To correct for differences in different corticosteroid therapies, treatment class was homogenized so that systemic corticosteroid use was considered when dosages equivalent to >24 mg/day methylprednisone or >30 mg/day prednisone were given acutely on ICU admission, as reported in previous studies [52]. Thirdly, in spite of the fact that microbiological confirmation based on current Infectious Disease Society of America (IDSA)/American Thoracic Society (ATS) guidelines [53] would be preferable, bronchoscopic procedures were not performed routinely because of severe hypoxemia complicating ARDS (H1N1)v episode and safety concerns regarding generation of aerosols. Finally, dosing of oseltamivir was left to the discretion of the attending physician and was not standardized. It is crucial to note that underdosing is a common problem in patients with severe sepsis and mechanical ventilation who have a high volume of distribution and low enteral absorption [54]. Ariano et al. [55] recently reported that dosage of 75 mg twice-daily achieved plasma levels that were comparable to those in ambulatory patients and were far in excess of concentrations required to maximally inhibit neuraminidase activity of the virus.
There is little definitive evidence of either benefit or harm from early corticosteroid use as routine adjunctive treatment in patients affected by pandemic (H1N1)v influenza A infection. Nevertheless, the results drawn from this study show that such early use did not result in better outcomes and may be associated with increased risk of superadded infections.
References
Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martínez S, Ferrer M, Avellanas M, Granada R, Maraví-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto del Portillo I, Galván B, León-Gil C: H1N1 SEMICYUC Working Group (2009) Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care 13:R148
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA: Canadian Critical Care Trials Group H1N1 Collaborative (2009) Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 302:1872–1879
The ANZIC Influenza Investigators (2009) Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med 361:1925–1934
Estenssoro E, Ríos FG, Apezteguía C, Reina R, Neira J, Ceraso DH, Orlandi C, Valentini R, Tiribelli N, Brizuela M, Balasini C, Mare S, Domeniconi G, Ilutovich S, Gomez A, Giuliani J, Barrios C, Valdez P (2010) Pandemic 2009 Influenza A(H1N1) in Argentina: a study of 337 patients on mechanical ventilation. Am J Respir Crit Care Med. doi:10.1164/rccm.201001-0037OC
Davis WB, Wilson HE, Wall RL (1986) Eosinophilic alveolitis in acute respiratory failure. A clinical marker for a non-infectious etiology. Chest 90:7–10
Luce JM, Montgomery AB, Marks JD, Turner J, Metz CA, Murray JF (1988) Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. Am Rev Respir Dis 138:62–68
Bernard GR, Luce JM, Sprung CL, Rinaldo JE, Tate RM, Sibbald WJ, Kariman K, Higgins S, Bradley R, Metz CA (1987) High dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med 317:1565–1570
Calfee CS, Matthay MA (2007) Nonventilatory treatments for acute lung injury and ARDS. Chest 131:913–920
Meduri GU, Marik PE, Chrousos GP, Pastores SM, Arlt W, Beishuizen A, Bokhari F, Zaloga G, Annane D (2008) Steroid treatment in ARDS: a critical appraisal of the ARDS network trial and the recent literature. Intensive Care Med 34:61–69
Agarwal R, Nath A, Aggarwal AN, Gupta D (2007) Do glucocorticoids decrease mortality in acute respiratory distress syndrome? A meta-analysis. Respirology 12:585–590
Tang BM, Craig JC, Eslick GD, Seppelt I, McLean AS (2009) Use of corticosteroid in acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med 37:1594–1603
Webb SAR, Seppelt IM, for the ANZIC Influenza Investigators (2009) Pandemic (H1N1) 2009 influenza (“swine flu”) in Australian and New Zealand intensive care. Crit Care Resusc 11:170–172
Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramírez P, Martin-Loeches I, Varillas D, Gallegos MC, Serón C, Micheloud D, Gomez JM, Tenorio-Abreu A, Ramos MJ, Molina ML, Huidobro S, Sanchez E, Gordón M, Fernández V, Del Castillo A, Marcos MA, Villanueva B, López CJ, Rodríguez-Domínguez M, Galan JC, Cantón R, Lietor A, Rojo S, Eiros JM, Hinojosa C, Gonzalez I, Torner N, Banner D, Leon A, Cuesta P, Rowe T, Kelvin DJ (2009) Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care 13:R201
Domínguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, De la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernández M, Stewart TE, Fowler RA (2009) Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 302:1880–1887
WHO Guidelines for pharmacological management of pandemic Influenza A(H1N1) 2009 and other Influenza viruses. http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf
Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza, Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, Kumar A, Lim M, Shindo N, Penn C, Nicholson KG (2010) Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 362:1708–1719
Salluh JI, Póvoa P (2010) Corticosteroids for H1N1 associated acute lung injury: is it just wishful thinking? Intensive Care Med 36:1098–1099
Quispe-Laime AM, Bracco JD, Barberio PA, Campagne CG, Rolfo VE, Umberger R, Meduri GU (2010) H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med 36:33–41
Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses. World Health Organization, June 2009. http://www.who.int/csr/resources/publications/SwineInfluenza_infectioncontrol.pdf
Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ (2009) Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 374:451–458
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG (2007) Infectious Diseases Society of America; American Thoracic Society: Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community—acquired pneumonia in adults. Clin Infect Dis 44(suppl 2):S27–S72
Cate TR (2001) Viral pneumonia due to influenza and parainfluenza viruses and adenoviruses. In: Marrie J Community-acquired pneumonia. Kluwer, New York, pp 593–616
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative workgroup (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8:R204–R212
Obesity and overweight (http://www.who.int/dietphysicalactivity/publications/facts/obesity/en. Accesed 20th March 2010)
Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR (2005) SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med 31:1336–1344
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR (2005) SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 31:1345–1355
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-Related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22:707–710
Chrousor GP (1995) The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332:1351–1362
Annane D, Sébille V, Troché G, Raphaël JC, Gajdos P, Bellissant E (2000) A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA 283:1038–1045
Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, Keh D, Briegel J, Beishuizen A, Dimopoulou I, Tsagarakis S, Singer M, Chrousos GP, Zaloga G, Bokhari F, Vogeser M, American College of Critical Care Medicine (2008) Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Crit Care Med 36:1937–1949
Montón C, Ewig S, Torres A, El-Ebiary M, Filella X, Rañó A, Xaubet A (1999) Role of glucocorticoids on inflammatory response in nonimmunosuppressed patients with pneumonia: a pilot study. Eur Respir J 14:218–220
Barnes PJ (2006) Corticosteroid effects on cell signalling. Eur Respir J 27: 413–426
Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU (2009) Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA 301:2362–2375
Minneci PC, Deans KJ, Eichacker PQ, Natanson C (2009) The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect 15:308–318
Sligl WI, Milner DA Jr, Sundar S, Mphatswe W, Majumdar SR (2009) Safety and efficacy of corticosteroids for the treatment of septic shock: a systematic review and meta-analysis. Clin Infect Dis 49:93–101
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(2):S27–S72
Confalonieri M, Urbino R, Potena A, Piattella M, Parigi P, Puccio G, Della Porta R, Giorgio C, Blasi F, Umberger R, Meduri GU (2005) Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med 171:242–248
Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG (2010) Efficacy of corticosteroid in community-acquired pneumonia—a randomized double blinded clinical trial. Am J Respir Crit Care Med. doi:10.1164/rccm.200905-0808OC
Summary of probable SARS cases with onset of illness from 1 November 2002 to 7 August 2003. [http://www.who.int/csr/sars/country/country2003_08_15.pdf]
Zhong NS, Zheng BJ, Li YM, Poon Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris Guan Y (2003) Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 362:1353–1358
CDC SARS Investigative Team (2003) Update: outbreak of severe acute respiratory syndrome–worldwide, 2003. MMWR Morb Mortal Wkly Rep 52:269–272
Cameron MJ, Bermejo-Martin JF, Danesh A, Muller MP, Kelvin DJ (2008) Human immunopathogenesis of severe acute respiratory syndrome (SARS). Virus Res 133:13–19
Cameron MJ, Ran L, Xu L, Danesh A, Bermejo-Martin JF, Cameron CM, Muller MP, Gold WL, Richardson SE, Poutanen SM, Willey BM, DeVries ME, Fang Y, Seneviratne C, Bosinger SE, Persad D, Wilkinson P, Greller LD, Somogyi R, Humar A, Keshavjee S, Louie M, Loeb MB, Brunton J, McGeer AJ, Canadian SARS Research Network, Kelvin DJ (2007) Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol 81:8692–8706
de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar (2006) Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 12:1203–1207
Li XW, Jiang RM, Guo JZ (2003) Glucocorticoid in the treatment of severe acute respiratory syndrome patients: a preliminary report. Chin J Intern Med 42:378–381
Tsang OT, Chau TN, Choi KW, Tso EY, Lim W, Chiu MC, Tong WL, Lee PO, Lam BH, Ng TK, Lai JY, Yu WC, Lai ST (2003) Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerg Infect Dis 9:1381–1387
Lee N, Allen Chan KC, Hui DS, Ng EK, Wu A, Chiu RW, Wong VW, Chan PK, Wong KT, Wong E, Cockram CS, Tam JS, Sung JJ, Lo YM (2004) Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol 31:304–309
Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J, CORTICUS Study Group (2008) Hydrocortisone therapy for patients with septic shock. N Engl J Med 358:111–124
Moreno R, Sprung C, Annane D, Keh D, Singer M, Briegel J, Freivogel K, Weiss Y, Benbenishty J, Kalenka A, Forst H, Laterre P, Reinhart K, Cuthberson B, Payen D (2007) Organ dysfunction/failure in patients with septic shock: results of the CORTICUS study. Intensive Care Med 33:S186
Dehoux MS, Boutten A, Ostinelli J, Seta N, Dombret MC, Crestani B, Deschenes M, Trouillet JL, Aubier M (1994) Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med 150:710–716
Garcia-Vidal C, Calbo E, Pascual V, Ferrer C, Quintana S, Garau J (2007) Effects of systemic steroids in patients with severe community-acquired pneumonia. Eur Respir J 30:951–956
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. (2005) Am J Respir Crit Care Med 171:388–416
Pea F, Viale P (2009) Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock—does the dose matter? Crit Care 13:214
Ariano RE, Sitar DS, Zelenitsky SA, Zarychanski R, Pisipati A, Ahern S, Kanji S, Rello J, Kumar A (2010) Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ 182:357–363
Conflict of interest
Authors declare no conflict of interest regarding the present manuscript.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Endorsed by the European Critical Care Research Network (ECCRN) of the European Society of Intensive Care Medicine (ESICM).
ESICM H1N1 Registry Contributors are listed in the Appendix.
Abstract selected as oral presentation in 23rd ESICM Annual Congress 2010.
Appendices
Appendix
ESICM H1N1 Registry Contributors
National coordinators:
Spain (Alejandro Rodriguez)
Portugal (Ricardo Matos)
Italy (Maurizia Capuzzo)
UK (Andrew Rhodes)
Colombia (Mario Villabon)
Argentina (Rosa Reina, Carina Balasini)
Ecuador (Diego Barahona)
Ireland (Brian Marsh)
Brazil (Eliezer Silva)
Norway (Haans Flaaten)
Iceland (Gisli Sigurdsson)
Czech Republic (Zykova Ivana, Vladimir Cerny)
Germany (Michael Quintel, Tobias Welte)
Peru (Manuel Mayorga)
France (Georges Offenstadt, Bertrand Guidet, Jean-Daniel Chiche)
Israel (Phillip Levin)
Switzerland (Hans-Ulrich Rothen)
Hong Kong (Charles Gomersall)
Iran (Seyed Mohammadreza Hashemian)
Greece (Constantine Katsanoulas, Heleni Mouloudi)
India (Farhad Kapadia)
Austria (Andreas Valentin)
Sweden (Goran Hedenstierna)
Denmark (Anders Perner)
Chile (Guillermo Bugedo)
Finland (Esko Ruokonen)
Spain
Jordi Rello, Antoni Soriano Arandes, Thiago Lisboa, Alejandro Rodriguez, Ignacio Martin-Loeches (Joan XXIII Universitary Hospital), Juan C. Montejo, Ramón Peñíscola (Hospital Universitario 12 De Octubre), Cecilia Hermosa, Federico Gordo (Hospital Del Henares), Jaime Latour (H General Universitario De Elche), Loreto Vidaur (Hospital Donostia), Manuel Alvarez-Gonzalez (Infanta Cristina), Luis Alvarez-Rocha (Complexo Hospitalario Universitario A Coruña), Ana De Pablo (Hospital Del Sureste), Cristina Ferri, Lopez De Arbina Martinez (Hospital Josep Trueta), Cortés Cânones (Complexo Hospitalario Ourense), Josu Insausti (Hospital De Navarra), Jose Cambronero (Hospital Universitario Príncipe De Asturias), Beatriz Galvan (H U La Paz), José Luna (Hospital De Tortosa Verge De La Cinta), Rafael Blancas (Hospital Del Tajo), Carmen Garcia (Infanta Elena Hospital), Rafael Sierra (Puerta Del Mar University Hospital), Francisco Fernández Dorado (Centro Médico Delfos), Pablo Monedero (Clínica Universidad Navarra), Jose Llagunes (General Valencia), Pedro Cobo (Hospital Punta De Europa, Algeciras, Cádiz), Antonia Socias (Hospital Son Llàtzer), Rafael Leon-Lopez (Hospital Universitario Reina Sofia), Elisabeth Esteban (Hospital Sant Joan De Déu), Marquina Lacueva (San Jorge), Monica Magret (Sant Joan Reus), Frutos Del Nogal (Severo Ochoa).
Portugal
Alexandra Dinis (UCIP, Hospital Pediátrico, CH de Coimbra), Anabela Bártolo (UCI, CH do Alto Ave, Guimarães), Armindo Ramos (UCI, Hospitais Privados de Portugal, Cascais), Carlos Franca (SMI, CH de Lisboa Norte), Celso Estevens (UCI, Hospital de Faro), Cristina Granja (UCIM, Hospital Pedro Hispano, Matosinhos), Custódio Fidalgo (UCI, Hospital de Santarém), Eduardo Almeida (UCI, Hospital Garcia de Orta, Almada), Estevão Lafuente (UCI, CH Tâmega e Sousa, Penafiel), Fernando Rua (SCI, CH do Porto), Francisco Esteves (UCI, CH Trás-os-Montes e Alto Douro, Vila Real), José Clemente (UCI, Hospital Nossa Senhora do Rosário, Barreiro), José Júlio Nóbrega (SMI, CH do Funchal), José Manuel Pereira (UCIPG, Hospital S. João, Porto), José Pedro Moura (UCI, CH do Alto Minho, Viana Do Castelo), Luís Paulo Trindade E Silva (ULS, Hospital Sousa Martins, Guarda), Luís Telo (UCIR, CH de Lisboa Norte), Lurdes Santos (UCIDI, Hospital S. João, Porto), Maria José Pedrosa (UCI, Hospital de Santo André, Leiria), Maria Oliveira, Margarida Resende (Hospital de Curry Cabral, Lisboa), Nuno Catorze (UCIP, CH Médio Tejo, Abrantes), Paula Coutinho (UCI, CH de Coimbra), Rosa Ribeiro (UCI, CH de Setúbal), Rui Moreno, Isabel Miranda, Ricardo Matos (UCIP, CH de Lisboa Central), Teresa Cardoso (UCIP, CH do Porto), Vítor Branco (UCI, CH da Cova da Beira, Covilhã).
Italy
Giacomo Bellani (San Gerardo Hospital-University Of Milan Bicocca), Ros Urbino (Ospedale Molinette Torino), Adriano Peris (Careggi Teaching Hospital), Alessandro Amatu (Fondazione IRCCS Policlinico San Matteo, Rianimazione), Giorgio Berlot (Cattainara (Trieste)), Federico Capra Marzani (Fondazione IRCCS Policlinico S. Matteo), Ulisse Corbanese (Rianimazione—Ospedale S. Maria dei Battuti—Conegliano), Antonio David (A.O.U. Policlinico “G. Martino” Messina), Paolo Chiarandini (AOU—S. Maria della Misericordia), Francesco Della Corte (ASO Maggiore Novara), Maria Luisa Caspani (Fondazione Ospedale Maggiore Policlinico, Mangiagalli E Regina Elena), Conio Alessandra (Ospedale Infantile Regina Margherita), Valerio Mangani (S. Giovanni Di Dio Hospital), Romano Tetamo (Arnas Civico), Andrea Wolfler (Children’s Hospital Buzzi), Giuseppe Tappatà (Macerata), Vivaldi Nicoletta (ASO “SS Antonio e Biagioe C. Arrigo” Alessandria), Maurizia Capuzzo (Azienda Ospedaliero-Universitaria di Ferrara), Guido Bertolini (GiViTI Coordinating Center), Lorella Pelagalli (National Cancer Institute Rome), Alexandre Molin (Ospedale San Martino), Massimo Girardis (Policlinico Di Modena), Giuseppe Gristin (S. Camillo Hospital, Rome).
UK
Rupert Pearse, Ammy Lam (Barts and the London NHS Trust), Andrew Rhodes (St. George’s Hospital), Ian Crabb (Gloucester), Rebecca Cusack (Southampton), Rhiannon Jackson (Frimley Park Hospital NHS Trust), Chithambaram Veerappan (The Royal Oldham Hospital, Oldham), Craig Whiteley, Tony Ware (Guy’s And St. Thomas’ NHS Foundation Trust), Stephan Dr. Krueper (University Hospital Of North Staffordshire NHS Trust), Caleb Mckinstry (Cheltenham Genera Hospital), Andrew Ferguson (Craigavon Area Hospital), Francesca Rubulotta (St. Mary).
Colombia
Mario Villabon (Hospital De San Jose Bogota), Erick Valencia (Sagrado Corazon).
Argentina
Susana Gonzalez (Hospital Pirovano), Carina Balasini (San Martín La Plata), Victor Cevallos (Hospital Velez Sarsfield), Alan Zazu (Clínica De Especialidades), Jeronimo Nahuel Chaparro Fresco (HIGA Rossi), Gabriel Galindez (Hospital Alejandro Korn), Rosa Reina (Hospital San Martín), Cecilia Barrios (Sanatorio Franchin), Carlos Lovesio (Sanatorio Parque).
Ecuador
Diego Barahona, Boris Villamagua, Mario Cadena (Hospital Eugenio Espejo), Estuardo Salgado (Clínica La Merced), Marìa Fernanda García (Hospital Eugenio—Neumologia) Gustavo Paredes (Hospital Del Sur).
Ireland
Maria Donnelly (Adelaide And Meath Hospital Dublin), Brian Marsh (Mater Misericordiae University Hospital), Donall O’Croinin (Mercy University Hospital), John Bates (UCHG), Niall Kavanagh (St. Luke’s General Hospital), Brian O’Brien (Waterford Regional), Rob Plant (Cork University Hospital), Michael Scully (Our Lady Of Lourdes), Rachel Farragher (Portiuncula Hospital).
Brazil
Louise Oliveira (Centro Hospitalar Unimed), Sergio Mataloun (UNISA—Hospital Geral Do Grajaú), Vicente Souza Dantas, Luiz Simvoulidis (Hospital Pasteur), Péricles Duarte (Hosp. São Lucas-FAG), Cintia Grion (Hospital Universitário De Londrina), Almir Germano (Hospital Universitario De Maringa).
Norway
Jon Henrik Laake (Rikshospitalet Medical Centre), Elin Helset (Oslo University Hospital, Ulleval), Dagny Klausen (Haugesund Sjukehus Helse Fonna), Hans Flaatten (Haukeland University Hospital), Kari Bruheim (St. Olavs Hospital).
Iceland
Bjarki Kristinsson (Landspitali), Sigurdur E. Sigurdsson (FSA).
Czech Republic
Jan Hrubý (KARIM VFN), Radka Valkova (ICU Infection’s Department, Masaryk’s Hospital Usti Nad Labem), Robert Janda (Karlovy Vary), Ivana Zykova (Krajska Nemocnice Liberec).
Germany
Andrea Kernchen (University Hospital Of Goettingen), Frank Bloos (University Hospital Jena), Simone Rosseau (Charité), Jens Krassler (Fachkrankenhaus Coswig GmbH), Frank Fischer (Klinikum Forchheim).
Peru
Abel Arroyo-Sanchez (Hospital Victor Lazarte Echegaray), Alejandro Barrionuevo Poquet (Hospital Nacional Carlos Alberto Seguin Escobedo), Ivan Ramos Palomino (Clínica San Gabriel), Fabiola Rafael (HCFAP), Juan Salasfoch (Hospital Regional Docente).
France
Gregory Dubar (Foch), Jean-Marie Tonnelier (University Hospital Of Brest), Saber Barbar (CHU Dijon), Murielle Dobrzynski (CHU Morvan), Alexandre Mignon (Hopital Cochin).
Israel
Daniel Jakobson (Barzilai MC), Moti Klein (Soroka Medical Center), Eran Segal (Assuta Medical Center), Yaron Barlavie (Rambam Hospital), Moshe Hersch (Shaare Zedek Medical Center).
Switzerland
Zule Sicardi Salomón (Astrid Lindgren Childerns Hospital), Hervé Zender (Hôpital Neuchâtelois, La Chaux-De-Fonds), Hans U. Rothen (Dept. Of Intensive Care Medicine, Bern University Hospital).
Hong Kong
Charles Gomersall (Prince Of Wales), Kenny Chan (Pamela Youde Nethersole Eastern Hospital), Tom Buckley (Princess Margaret Hospital).
Iran
Seyed Mohammadreza Hashemian [Nritld (Masih Daneshvari)].
Serbia and Montenegro
Uros Batranovic (Institute For Pulmonary Diseases Of Vojvodina), Ilons Schaffer (Hospital Health Center Kikinda), Jelena Sretkovic (KC Kragujevac).
Greece
Despoina Koulenti (Critical Care Department Attikon University Hospital, Athens), Eleni Mouloudi (Hippokrateion General Hospital Thessaloniki), Phyllis-Maria Clouva-Molyvdas (ICU Thriassio Hospital Of Eleusis).
India
Mohan Gurjar (Sanjay Gandhi Post Graduate Institute Of Medical Sciences), Deepak Vijayan (Kerala Institute Of Medical Sciences).
Austria
Georg Hinterholzer (Kaiser Franz Josef Spital Vienna, 1. Med), Alexander Kulier (Medical University Of Graz).
The Netherlands
Carin Verlaat (Universitary Medical Center Nijmegen), Dirk Ebel (Slingeland Ziekenhuis).
Sweden
Johan Persson (Lund University Hospital), Sten Walther (THIVA, US, Linköping), Per Petersen (Uddevalla).
Belgium
Walter Swinnen (Az Sint-Blasius), Vincent Collin (Cliniques De L’Europe, Saint-Michel).
Denmark
Hanne Olsen (OUH, Svendborg Sygehus).
Bolivia
Patricio Gutierrez (Hospital Materno Infantil—Caja Nacional de Salud).
Bosnia and Herzegovina
Guillaume Thiery (Clinical Center University Sarajevo).
Chile
Guillermo Bugedo (Universidad Católica).
Finland
Heikki Laine (Central Hospital of Mikkeli).
Lithuania
Arvydas Rumba (Clinics of Kaunas University of Medicine).
Mauritius
Sundaresan Maiyalagan (Fortis Clinique Darne).
Qatar
Jose Clemente (Centro Hospitalar Barreiro Montijo).
Vietnam
Thinh Bui (Trung Vuong Hospital).
Rights and permissions
About this article
Cite this article
Martin-Loeches, I., Lisboa, T., Rhodes, A. et al. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 37, 272–283 (2011). https://doi.org/10.1007/s00134-010-2078-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2078-z