Abstract.
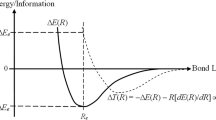
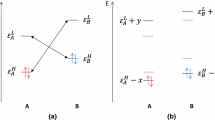
New and useful aspects of chemical reactivity as described by reactivity indexes and used in connection with the maximum hardness and minimum polarizability principles (MHP and MPP, respectively) are discussed and illustrated for two classical reactions in organic chemistry. They include the Beckmann rearrangement and the condensation reactions of α-amino acids. The MPP appears as a more general rule than the MHP. Another relevant result is related to the usefulness of both empirical reactivity rules to predict the most probable reaction mechanism among two different pathways displaying very close values in activation energy (competitive pathways). This is illustrated for the condensation reaction of a series of α-amino acids: while the accepted stepwise route follows both the MHP and MPP rules, the alternative concerted channel does not, yet its associated activation energy is slightly lower than that corresponding to the nonconcerted reaction mechanism.
Similar content being viewed by others
Acknowledgments.
This work received financial support from Fondecyt, grants 2000092, 1010649 and 1030548. B.G. is a DAAD fellow.
Author information
Authors and Affiliations
Corresponding author
Additional information
From the Proceedings of the 28th Congresco de Quimicos Teóricos de Expresión Latina (QUITEL 2002)
Rights and permissions
About this article
Cite this article
Gómez, B., Fuentealba, P. & Contreras, R. The maximum hardness and minimum polarizability principles as the basis for the study of reaction profiles. Theor Chem Acc 110, 421–427 (2003). https://doi.org/10.1007/s00214-003-0497-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00214-003-0497-4