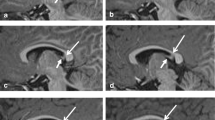
Abstract
Left visual neglect is a dramatic neurological condition that impairs awareness of left-sided events. Neglect has been classically reported after strokes in the territory of the right middle cerebral artery. However, the precise lesional correlates of neglect within this territory remain discussed. Recent evidence strongly suggests an implication of dysfunction of large-scale perisylvian networks in chronic neglect, but the quantitative relationships between neglect signs and damage to white matter (WM) tracts have never been explored. In this prospective study, we used diffusion tensor imaging (DTI) tractography in twelve patients with a vascular stroke in the right hemisphere. Six of these patients showed signs of neglect. Nonparametric voxel-based comparisons between neglect and controls on fractional anisotropy maps revealed clusters in the perisylvian WM and in the external capsule. Individual DTI tractography identified specific disconnections of the fronto-parietal and fronto-occipital pathways in the neglect group. Voxel-based correlation statistics highlighted correlations between patients’ performance on two visual search tasks and damage to WM clusters. These clusters were located in the anterior limb of the internal capsule and in the WM underlying the inferior frontal gyrus, along the trajectory of the anterior segment of the arcuate fasciculus (asAF). These results indicate that chronic visual neglect can result from, and correlate with, damage to fronto-parietal connections in the right hemisphere, within large-scale cortical networks important for orienting of spatial attention, arousal and spatial working memory.





Similar content being viewed by others
Notes
These correlations should be interpreted with caution because they are calculated across groups with non-overlapping performance (with and without neglect) that may drive an artefactual correlation. However, visual inspection of the graphs in Fig. 5 shows that patients with more severe micro-structural damage tend to show more severe neglect on cancellation tests.
In order to avoid acute ischemic MRI artefacts such as cell swelling or cytotoxic oedema (see Sotak 2002), we established a minimum necessary time interval of 3 weeks between stroke and DT-MR acquisition; as a consequence, many patients tested behaviourally could not be included because they had left the hospital before any DTI sequence could be acquired. Time interval between stroke onset and MRI was chosen on the basis of several studies indicating an initial increase of FA at the acute stage due to cell swelling. At the sub-acute and chronic stages, there is a decreasing of FA in the lesion, due to wallerian degeneration (Thomalla et al. 2005). This decrease could remain significant (compared to the FA in the homologous controlateral lesion) even 6 months after the stroke (Sotak 2002). The second raison was the probability of frequent consecutive oedema at the acute stage, which could have disturbed the sensitivity of the DTI sequence to the lesion.
References
Albert ML (1973) A simple test of visual neglect. Neurology 23:658–664
Ashburner J, Friston KJ (2000) Voxel-based morphometry—the methods. NeuroImage 11:805–821
Bartolomeo P (2006) A parietofrontal network for spatial awareness in the right hemisphere of the human brain. Arch Neurol 63:1238–1241
Bartolomeo P (2007) Visual neglect. Curr Opin Neurol 20:381–386
Bartolomeo P, Chokron S (1999) Egocentric frame of reference: its role in spatial bias after right hemisphere lesions. Neuropsychologia 37:881–894
Bartolomeo P, Chokron S (2002) Orienting of attention in left unilateral neglect. Neurosci Biobehav Rev 26:217–234
Bartolomeo P, D’Erme P, Gainotti G (1994) The relationship between visuospatial and representational neglect. Neurology 44:1710–1714
Bartolomeo P, Thiebaut de Schotten M, Doricchi F (2007) Left unilateral neglect as a disconnection syndrome. Cereb Cortex 17:2479–2490
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Bates E, Wilson S, Saygin A, Dick F, Sereno M, Knight RT, Dronkers N (2003) Voxel-based lesion-symptom mapping. Nat Neurosci 6:448–450
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757
Binder JR, Marshall R, Lazar R, Benjamin J, Mohr JP (1992) Distinct syndromes of hemineglect. Arch Neurol 49:1187–1194
Bird C, Malhotra P, Parton A, Coulthard EJ, Rushworth MF, Husain M (2006) Visual neglect after right posterior cerebral artery infarction. J Neurol Neurosurg Psychiatr 77:1008–1012
Büchel C, Friston K (1997) Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb Cortex 7:768–778
Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K (2006) White matter fiber tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. NeuroImage 29:1092–1105
Catani M, Ffytche D (2005) The rises and falls of disconnection syndromes. Brain 128:2224–2239
Catani M, Mesulam M (2008) The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex 44:953–961
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132
Catani M, Howard RJ, Pajevic S, Jones DK (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage 17:77–94
Catani M, Jones DK, Donato R, Ffytche D (2003) Occipito-temporal connections in the human brain. Brain 126:2093–2107
Catani M, Jones DK, Ffytche D (2005) Perisylvian language networks of the human brain. Ann Neurol 57:8–16
Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK (2007) Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104:17163–17168
Ciccarelli O, Toosy AT, Parker GJ, Wheeler-Kingshott CA, Barker GJ, Miller DH, Thompson AJ (2003) Diffusion tractography based group mapping of major white-matter pathways in the human brain. NeuroImage 19:1545–1555
Committeri G, Pitzalis S, Galati G, Patria F, Pelle G, Sabatini U, Castriota-Scanderbeg A, Piccardi L, Guariglia C, Pizzamiglio L (2007) Neural bases of personal and extrapersonal neglect in humans. Brain 130:431–441
Corbetta M, Shulman G (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:215–229
Corbetta M, Kincade M, Lewis C, Snyder A, Sapir A (2005) Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci 8:1603–1610
Corbetta M, Patel G, Shulman G (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324
Coulthard EJ, Nachev P, Husain M (2008) Control over conflict during movement preparation: role of posterior parietal cortex. Neuron 58:144–157
Crosby EC, Humphrey T, Lauer EW (1962) Correlative anatomy of the nervous system. Macmillian Co., New York
Dell’Acqua F, Scifo P, Rizzo G, Catani M, Simmons A, Scotti G, Fazio F (2010) A modified damped Richardson–Lucy algorithm to reduce isotropic background effects in spherical deconvolution. NeuroImage 49:1446–1458
Doricchi F, Angelelli P (1999) Misrepresentation of horizontal space in left unilateral neglect: role of hemianopia. Neurology 52:1845–1852
Doricchi F, Tomaiuolo F (2003) The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport 14:2239–2243
Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P (2008) White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex 44:983–995
Duffau H (2009) Does post-lesional subcortical plasticity exist in the human brain? Neurosci Res 65:131–135
Duffau H, Capelle L, Sichez J, Faillot T, Abdennour L, Law Koune JD, Dadoun S, Bitar A, Arthuis F, Van Effenterre R, Fohanno D (1999) Intra-operative direct electrical stimulations of the central nervous system: the Salpêtrière experience with 60 patients. Acta Neurochir 141:1157–1167
Friston KJ, Büchel C (2000) Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci USA 97:7591–7596
Gaffan D, Hornak J (1997) Visual neglect in the monkey. Representation and disconnection. Brain 120:1647–1657
Gainotti G, D’Erme P, Bartolomeo P (1991) Early orientation of attention toward the half space ipsilateral to the lesion in patients with unilateral brain damage. J Neurol Neurosurg Psychiatr 54:1082–1089
Gauthier L, Dehaut F, Joanette Y (1989) The bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol 11:49–53
Golay L, Schnider A, Ptak R (2008) Cortical and subcortical anatomy of chronic spatial neglect following vascular damage. Behav Brain Funct 4:43
Good CD (2001) Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage 14:685–700
Goodlett CB, Fletcher P, Gilmore JH, Gerig G (2009) Group analysis of DTI fiber tract statistics with application to neurodevelopment. NeuroImage 45:S133–S142
Gosh S, Rao PS, De G, Majumder PP (2007) A nonparametric regression-based linkage scan of rheumatoid factor-IgM using sib-pair squared sums and differences. BMC Proc 1:S99
Hayasaka S, Nichols T (2003) Validating cluster size inference: random field and permutation methods. NeuroImage 20:2343–2356
Heilman KM, Watson RT, Bower D, Valenstein E (1983) Right hemisphere dominance for attention. Rev Neurol (Paris) 139:15–17
Holmes AP, Blair RC, Watson JD, Ford I (1996) Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab 16:7–22
Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC (1998) Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22:324–333
Husain M, Kennard C (1996) Visual neglect associated with frontal lobe infarction. J Neurol 243:652–657
Husain M, Nachev P (2007) Space and the parietal cortex. Trends Cogn Sci 11:30–36
Husain M, Rorden C (2003) Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci 4:26–36
Jones DK, Symms MR, Cercignani M, Howard RJ (2005) The effect of filter size on VBM analyses of DT-MRI data. NeuroImage 26:546–554
Karnath HO, Fruhmann Berger M, Küker W, Rorden C (2004) The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex 14:1164–1172
Karnath HO, Rorden C, Ticini LF (2009) Damage to white matter fiber tracts in acute spatial neglect. Cereb Cortex 19:2331–2337
Kinsbourne M (1993) Orientational bias model of unilateral neglect: evidence from attentional gradients within hemispace. In: Robertson IH, Marshall JC (eds) Unilateral neglect: clinical and experimental studies. Lawrence Erlbaum Associates, Hove, pp 63–86
Koch G, Oliveri M, Cheeran B, Ruge D, Lo Gerfo E, Salerno S, Torriero S, Marconi B, Mori F, Driver J, Rothwell JC, Caltagirone C (2008) Hyperexcitability of parietal-motor functional connections in the intact left-hemisphere of patients with neglect. Brain 131:3147–3155
Kunimatsu A, Aoki S, Masutani Y, Abe O, Hayashi N, Mori H, Masumoto T, Ohtomo K (2004) The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci 3:11–17
Leibovitch FS, Black SE, Caldwell CB, Ebert PL, Ehrlich LE, Szalai JP (1998) Brain-behavior correlations in hemispatial neglect using CT and SPECT: the Sunnybrook stroke study. Neurology 50:901–908
Losier BJ, Klein RM (2001) A review of the evidence for a disengage deficit following parietal lobe damage. Neurosci Biobehav Rev 25:1–13
Malhotra P, Parton AD, Greenwood R, Husain M (2006) Noradrenergic modulation of space exploration in visual neglect. Ann Neurol 59:186–190
McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B (1994) Network analysis of cortical visual pathways mapped with PET. J Neurosci 14:655–666
Mesulam M (1981) A cortical network for directed attention and unilateral neglect. Ann Neurol 10:309–325
Mesulam M (1985) Principles of behavioral neurology. F.A. Davis, Philadelphia
Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M (2003) The anatomy of visual neglect. Brain 126:1986–1997
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Nobre AC (2001) The attentive homunculus: now you see it, now you don’t. Neurosci Biobehav Rev 25:477–496
Park KC, Lee BH, Kim EJ, Shin MH, Choi KM, Yoon SS, Kwon SU, Chung CS, Lee KH, Heilman KM, Na DL (2006) Deafferentation-disconnection neglect induced by posterior cerebral artery infarction. Neurology 66:56–61
Pisella L, Rode G, Farnè A, Tilikete C, Rossetti Y (2006) Prism adaptation in the rehabilitation of patients with visuo-spatial cognitive disorders. Curr Opin Neurol 19:534–542
Posner MI, Walker JA, Friedrich FJ, Rafal RD (1984) Effects of parietal injury on covert orienting of attention. J Neurosci 4:1863–1874
Reep RL, Corwin JV, Cheatwood JL, Van Vleet TM, Heilman KM, Watson RT (2004) A rodent model for investigating the neurobiology of controlateral neglect. Cogn Behav Neurol 17:191–194
Robertson LC (2003) Binding, spatial attention and perceptual awareness. Nat Rev Neurosci 4:93–102
Robertson IH, Halligan PW, Bergego C, Homberg V, Pizzamiglio L, Weber E, Wilson BA (1994) Right neglect following right hemisphere damage? Cortex 30:199–213
Rodrigo S, Naggara O, Oppenheim C, Golestani N, Poupon C, Cointepas Y, Mangin JF, Le Bihan D, Meder JF (2007) Human subinsular asymmetry studied by diffusion tensor imaging and fiber tracking. AJNR Am J Neuroradiol 28:1526–1531
Rousseaux M, Beis JM, Pradat-Diehl P, Martin Y, Bartolomeo P, Bernati T, Chokron S, Leclercq M, Louis-Dreyfus A, Marchal F, Perennou D, Prairial C, Rode G, Samuel C, Sieroff E, Wiart L, Azouvi P (2001) Presenting a battery for assessing spatial neglect. Norms and effects of age, educational level, sex, hand and laterality. Rev Neurol (Paris) 157:1385–1400
Salmond CH, Ashburner J, Vargha-Khadem F, Connelly A, Gadian DG, Friston K (2002) Distributional assumptions in voxel-based morphometry. NeuroImage 17:1027–1030
Schmahmann JD, Pandya D (2006) Fiber pathways of the brain. Oxford University Press, New York
Shinoura N, Suzuki Y, Yamada R, Tabei Y, Saito K, Yagi K (2009) Damage to the right superior longitudinal fasciculus in the inferior parietal lobe plays a role in spatial neglect. Neuropsychologia 47:2600–2603
Singh-Curry V, Husain M (2009) The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia 47:1434–1448
Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols T, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ, Behrens TE (2007) Acquisition and voxelwise analysis of multisubject diffusion data with Tract-Based Spatial Statistics. Nat Protoc 2:499–504
Sotak CH (2002) The role of diffusion tensor imaging in the evaluation of ischemic brain injury—a review. NMR Biomed 15:561–569
Taoka T, Morikawa M, Akashi T, Miyasaka T, Nakagawa H, Kiuchi K, Kishimoto T, Kichikawa K (2009) Fractional anisotropy—threshold dependence in tract-based diffusion tensor analysis: evaluation of the uncinate fasciculus in Alzheimer disease. AJNR Am J Neuroradiol 30:1700–1703
Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Levy R, Dubois B, Bartolomeo P (2005) Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science 309:2226–2228
Thiebaut de Schotten M, Kinkingnéhun S, Delmaire C, Lehéricy S, Duffau H, Thivard L, Volle E, Levy R, Dubois B, Bartolomeo P (2008) Visualization of disconnection syndromes in humans. Cortex 44:1097–1103
Thiebaut de Schotten M, Ffytche D, Bizzi A, Dell’Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DGM, Catani M (2010) Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography, NeuroImage. doi:10.1016/j.neuroimage.2010.07.055
Thomalla G, Glauche V, Weiller C, Rother J (2005) Time course of wallerian degeneration after ischaemic stroke revealed by diffusion tensor imaging. J Neurol Neurosurg Psychiatry 76:266–268
Tryon WW (2001) Evaluating statistical difference, equivalence, and indeterminacy using inferential confidence intervals: an integrated alternative method of conducting null hypothesis statistical tests. Psychol Methods 6:371–386
Tryon WW, Lewis C (2008) An inferential confidence interval method of establishing statistical equivalence that corrects Tryon’s (2001) reduction factor. Psychol Methods 13:272–277
Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touze E, Chokron S, Meder JF, Levy R, Dubois B, Bartolomeo P (2008) Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry 79:598–601
Vallar G (2001) Extrapersonal visual unilateral spatial neglect and its neuroanatomy. NeuroImage 14:S52–S58
Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P (2010) Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain 133:880–894
Winkler AM, Nichols T, Glahn DC (2008) On non-normality, non-parametric tests and pooling permutations over space for voxel-based morphometry. Poster presented at the congress human brain mapping, Melbourne, Australia
Acknowledgments
Supported by grants from the AP-HP (interface programme) and the Université Pierre et Marie Curie, Paris 6 (Bonus Qualité Recherche) to PB.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Urbanski, M., Thiebaut de Schotten, M., Rodrigo, S. et al. DTI-MR tractography of white matter damage in stroke patients with neglect. Exp Brain Res 208, 491–505 (2011). https://doi.org/10.1007/s00221-010-2496-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2496-8