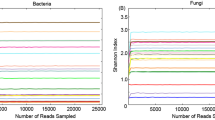
Abstract
Microbiota in the gastrointestinal tract (GIT) plays an essential role in the health and well-being of the host. With the exception of chickens, this area has been poorly studied within birds. The avian GIT harbours unique microbial communities. Birds require rapid energy bursts to enable energy-intensive flying. The passage time of feed through the avian GIT is only 2–3.5 h, and thus requires the presence of microbiota that is extremely efficient in energy extraction. This investigation has used high-throughput 16S rRNA gene sequencing to explore the GIT microbiota of the flighted bird, the Japanese quail (Coturnix japonica). We are reporting, for the first time, the diversity of bacterial phylotypes inhabiting all major sections of the quail GIT including mouth, esophagus, crop, proventriculus, gizzard, duodenum, ileum, cecum, large intestine and feces. Nine phyla of bacteria were found in the quail GIT; however, their distribution varied significantly between GIT sections. Cecal microbiota was the most highly differentiated from all the other communities and showed highest richness at an OTU level but lowest richness at all other taxonomic levels being comprised of only 15 of total 57 families in the quail GIT. Differences were observed in the presence and absence of specific phylotypes between sexes in most sections.






Similar content being viewed by others
References
Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ (2005) At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl Environ Microbiol 71(12):7724–7736
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Chapman J, Regan F (2011) Sebacic and succinic acid derived plasticised PVC for the inhibition of biofouling in its initial stages. J Appl Biomater Biomed 9(3):176–184
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57(Pt 10):2259–2261
Clench MH, Mathias JR (1995) The avian cecum: a review. Wilson Bull 107(1):93–121
DeBruyn JM, Fawaz MN, Peacock AD, Dunlap JR, Nixon LT, Cooper KE, Radosevich M (2013) Gemmatirosa kalamazoonesis gen. nov., sp. nov., a member of the rarely-cultivated bacterial phylum Gemmatimonadetes. J Gen Appl Microbiol 59(4):305–312
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72(7):5069–5072
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J (2014) An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2(1):6
Golian A, Maurice DV (1992) Dietary poultry fat and gastrointestinal transit time of feed and fat utilization in broiler chickens. Poult Sci 71(8):1357–1363
Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, Bushman FD, Knight R, Kelley ST (2011) Bayesian community-wide culture-independent microbial source tracking. Nat Methods 8(9):761–763
Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339(6123):1084–1088
Mead GC (1989) Microbes of the avian cecum: types present and substrates utilized. J Exp Zool Suppl 3:48–54
Pan D, Yu Z (2014) Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5(1):108–119
Raji AO, Alade NK, Duwa H (2014) Estimation of model parameters of the Japanese quail growth curve using Gompertz model. Archivos de Zootecnia 63 (243)
Sekelja M, Rud I, Knutsen SH, Denstadli V, Westereng B, Naes T, Rudi K (2012) Abrupt temporal fluctuations in the chicken fecal microbiota are explained by its gastrointestinal origin. Appl Environ Microbiol 78(8):2941–2948
Skrzypek T, Valverde Piedra JL, Skrzypek H, Wolinski J, Kazimierczak W, Szymanczyk S, Pawlowska M, Zabielski R (2005) Light and scanning electron microscopy evaluation of the postnatal small intestinal mucosa development in pigs. J Physiol Pharmacol 56(Suppl 3):71–87
Sommer F, Backhed F (2013) The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 11(4):227–238
Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ (2013) Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE 8(12), e84290
Stanley D, Hughes RJ, Moore RJ (2014) Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl Microbiol Biotechnol 98(10):4301–4310
Stanley D, Geier MS, Chen H, Hughes RJ, Moore RJ (2015) Comparison of fecal and cecal microbiotas reveals qualitative similarities but quantitative differences. BMC Microbiol 15(1):51
Su H, McKelvey J, Rollins D, Zhang M, Brightsmith DJ, Derr J, Zhang S (2014) Cultivable bacterial microbiota of northern bobwhite (Colinus virginianus): a new reservoir of antimicrobial resistance? PLoS ONE 9(6), e99826
Waite DW, Taylor MW (2014) Characterizing the avian gut microbiota: membership, driving influences, and potential function. Front Microbiol 5:223
Acknowledgments
We wish to thank Clive and Erika Wylie, the owners of Banyard Game Farms who provided the birds used in this study. We thank Honglei Chen for operating the Illumina MiSeq instrument. The data analysis was performed on Isaac Newton High Performance Computing System at Central Queensland University. We wish to acknowledge help from Jason Bell provided in all aspects of High Performance Computing as well as generous help we received from Tanka Prasai, Giselle Weegenaar, Ingrid Christiansen and Judy Couper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animal ethics approval for the present project was obtained from the Animal Ethics Committee of the Central Queensland University (A14/03-309).
Grants
This research was conducted within the Poultry CRC, established and supported under the Australian Government’s Cooperative Research Centres Program. Poultry CRC provides scholarship for Ngare Wilkinson.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3011 kb)
Rights and permissions
About this article
Cite this article
Wilkinson, N., Hughes, R.J., Aspden, W.J. et al. The gastrointestinal tract microbiota of the Japanese quail, Coturnix japonica . Appl Microbiol Biotechnol 100, 4201–4209 (2016). https://doi.org/10.1007/s00253-015-7280-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7280-z