Abstract
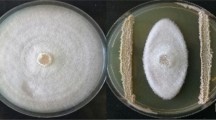
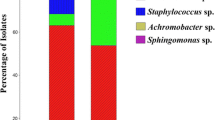
Phaseolus vulgaris is originally from the American continent. It is renowned as one of the preferred legume choice in the Peruvian market, due to its high content of nutrients. The Peruvian coast valleys are key-production areas for local varieties of the common bean crops. Soil-borne plant pathogens, however, favored by soil and environmental conditions, may reduce crop production. The aim of this study was to conduct a bio prospection of the antagonistic native bacteria of the north, south and central part of the coastal areas related to the common bean. A collection of 26 strains isolated from the rhizosphere of common bean plants showed high potential to control the growth of Sclerotinia, Fusarium and Rhizoctonia due to the production of both volatile and non-volatile organic compounds. Most of the strains were able to suppress fungal growth due to the presence of non-volatile organic compounds, such as hydrolytic enzymes, siderophores and antifungal lipopeptide production. Bacillus IcBac2.1 strain showed a remarkable ability to halt the majority of phytopathogens producing antifungal lipopeptides. The crude lipopeptides were soluble in polar solvents and remained stable at high temperatures and low pH. Strains were also able to inhibit fungal growth through volatile organic compounds. Alcaligenes TvPs2.4 and Pseudomonas TvPs1.6 showed the highest inhibition strength against the tested phytopathogens. Each strain produced 21 volatile organic compounds detected by SPME/GC–MS analysis. The compounds with the highest concentration were dimethyl disulfide and d-limonene. The 16S rRNA gene sequence confirmed that the strains were closely related to Bacillus, Paenibacillus, Achromobacter, Pseudomonas, Serratia and Alcaligenes.


Similar content being viewed by others
References
MINAGRI (Ministerio del Ambiente) (2018) Mapa nacional de ecosistemas del Peru. Memoria descriptiva. https://sinia.minam.gob.pe/normas/aprueban-mapa-nacional-ecosistemas-memoria-descriptiva-las-definiciones. Accessed 21 Dec 2018
Agrios GN (2011) Fitopatología. LIMUSA-Noriega Editores, second edition
Iqbal Z, Khan MA, Sharif M, Shah JH, Rehman MH, Javed K (2018) An automated detection and classification of citrus plant diseases using image processing techniques: a review. Comput Electron Agric 153:12–32
Ampt EA, Van Ruijven J, Raaijmakers JM et al (2019) Linking ecology and plant pathology to unravel the importance of soil-borne fungal pathogens in species-rich grasslands. Eur J Plant Pathol 154:141–156
Camarena M, Huaringa J, Mostacero N (2009). Innovación tecnológica para el incremento de la producción de frijol común (Phaseolus vulagaris L.). Primera Edición. Universidad Nacional Agraria La Molina—Consejo de Ciencia, Tecnología e Innovación Tecnológica.
Hillocks RJ, Cooper JE (2012) Hillocks integrated pest management—can it contribute to sustainable food production in Europe with less reliance on conventional pesticides? Outlook Agric 41(4):237–242
American Public Health Association (APHA) (1998) Standard methods for examination of water and waste water, 20th edn. EEUU, Washington, DC
American Public Health Association (APHA) (1975) Standard Methods for examination of water and waste water, 14th edn. EEUU, Washington, DC
Merck (1994) Manual de Medios de Cultivo. E Merck, Darmstandt, Alemania
Vessey J (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Barnett HL, Hunter BB (1998) Illustrated genera of imperfect fungi. American Phytopathological Society, St. Paul
Anguiz R, Martin C (1989) Anastomosis groups, pathogenicity, and other characteristics of Rhizoctonia solani isolated from potatoes in Peru. Plant Dis 73:199–201
Nelson P, Toussoun T, Marasas W (1993) Fusarium species: an illustrated manual for identification. Pennsylvania State University Press, New York
Calvo P, Ormeño E, Martínez E, Zúñiga D (2010) Characterization of Bacillus isolates of potato rhizosphere from andean soils of Peru and their potential PGPR characteristics. Braz J Microbiol 41:899–906
Zhu Z, Zhang GY, Luo Y, Ran W, Shen QR (2012) Production of lipopeptides by Bacillus amyloliquefaciens XZ-173 in solid state fermentation using soybean flour and rice straw as the substrate. Bioresour Technol 112:254–260
Sagar K, Bashir Y, Phukan MM, Konwar BK (2013) Isolation of lipolytic bacteria from waste contaminated soil: a study with regard to process optimization for lipase. IJSTR 2:214–218
Sudarshan SR, Mannivannan A, Narendhirakannan RT (2014) Screening of cellulase producing microorganisms from lake area containing water hyacinth for enzymatic hydrolysis of cellulose. J Adv Sci Res 5:23–30
Venant N, Pascal K, Ernest S (2013) Isolation of Bacillus strains from the rhizosphere of Tomato and their in vitro antagonistic effects against phytopathogenic fungi. GARJM 2:65–71
Castro R, Álvarez A, Machado E, Mendoza M, Gómez R, García P (2011) Caracterización de una quitinasa extracelular producida por Serratia sp. biomi-363706 usando quitina coloidal como sustrato. Revista Sociedad Química del Perú 77:101–108
Louden BC, Haarmann D, Lynne AM (2011) Use of blue agar CAS assay for siderophore detection. J Microbiol Biol Educ 12(1):51–53
Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Claus D, Berkeley RCW (1986) Genus Bacillus. In: Sneath PH, Mair N, Sharpe ME, Holt JG (eds) En Bergey’s Manual of Systematic Bacteriology, vol 2, 9th edn. Williams and Wilkins, Baltimore, pp 1105–1139
Calvo P, Raymundo LE, Zúñiga D (2008) A study of potato (Solanum tuberosum) crop rhizosphere microbial population in highland zones. Ecología Aplicada 7:141–148
Reyes I, Valery A (2007) Efecto de la fertilidad del suelo sobre la microbiota y la promoción del crecimiento (Zea mays L.) con Azotobacter spp. Bioagro 19:117–126
Compant S, Duffy B, Nowak J, Clement C, Barka E (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Fritze D (2004) Taxonomy of the genus Bacillus and related genera: the aerobic endospore-forming bacteria. Phytopathology 94:1245–1248
Memenza M, Mostacero E, Camarena F, Zuñiga D (2016) Disease control and plant growth promotion (PGP) of selected bacterial strains in Phaseolus vulgaris. In: Gonzales-Andres F, James E (eds) Biological nitrogen fixation and beneficial plant—microbe interactions. Springer, Cham, Switzerland, pp 237–245
Meena KR, Kanwar SS (2015) Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. BioMed Res Int. https://doi.org/10.1155/2015/473050
Guo Q, Dong W, Li S, Lu X, Wang P, Zhang X, Wang Y, Ma P (2014) Fengycin produced by Bacillus subtilis NCD-2 plays a major role in biocontrol of cotton seedling damping-off disease. Microbiol Res 169:533–540
Memenza-Zegarra M, Zúñuga-Dávila D (2019) Isolation and characterization of antifungal secondary metabolites produced by rhizobacteria from common bean. In: Zúñiga-Dávila D, González-Andrés F, Ormeño-Orrillo E (eds) Probiotics for agricultural systems advances in agronomic use. Springer, Cham, Switzerland, pp 141–153
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857
Wang H, Fewer DP, Holm L, Rouhiainen L, Sivonen K (2014) Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc Natl Acad Sci USA 111:9259–9264
Béchet M, Caradec T, Hussein W, Abderrahmani A, Chollet M, Leclère V, Dubois T, Lereclus D, Pupin M, Jacques P (2012) Structure, biosynthesis and properties of kurstakins, nonribosomal lipopeptides from Bacillus spp. Appl Microbiol Biotechnol 95:593–600
Stein (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions: Bacillus subtilis antibiotics. Mol Microbiol 56:845–857
Haggag W, Abd-El-Kareem F, Abou-Hussein S (2013) Bioprocessing of Brevibacillus brevis and Bacillus Polymyxa: a potential biocontrol agent of gray mould disease of strawberry fruits. IJEIT 3:509–518
Algam SA, Xie GL, Li B, Coosemans J, Liu B (2004) Comparative performance of Bacillus spp. in growth promotion and suppression of tomato bacterial wilt caused by Ralstonia solanacearum. J Zhejiang Univ 30:603–610
Chaurasia B, Pandey A, Palni LMS, Tribedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
Alström S (2001) Characteristics of bacteria from oil seed rape in relation to their biocontrol activity against Verticillium dahliae. J Phytopathol 149:57–64
Wheatley RE (2002) The consequences of volatile organic compound mediated bacterial and fungal interactions. A van Leeuw J Microb 81:357–364
Pichersky E, Noel J, Dudareva N (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311:808–811
Li Q, Ning P, Zheng I, Huang J (2010) Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biol Technol 58:157–165
Fujioka K, Gotoh H, Noumi T, Yoshida A, Noutoshi Y, Inagaki Y, Yamamoto M, Shiraishi T, Toyoda K (2015) Protection induced by volatile limonene against anthracnose disease in Arabidopsis thaliana. J Gen Plant Pathol 81:415–419
Giorgio A, De Stradis A, Lo Cantore P, Iacobellis NS (2015) Biocide effects of volatile organic compounds produced by potential biocontrol rhizobacteria on Sclerotinia sclerotiorum. Front Microbiol 6:1056
Acknowledgements
To Esperanza Martinez from the CCG-UNAM, Mexico for the critical review of the article and to Katty Ogata Gutiérrez for her support in the elaboration and analysis of the phylogenetic tree.
Funding
This research was supported by Programa Nacional de Innovación para la Competitividad y Productividad (Innovate Perú) [Contract 158-PNCIP-PIAP-2015] and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECyT) [Contract No. 009-2017].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MM-Z and DZ-D. The first draft of the manuscript was written by MM-Z and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Informed Consent
All authors were consent to participate in the elaboration and execution of this work. All authors are agreed with the content share in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Memenza-Zegarra, M., Zúñiga-Dávila, D. Bioprospection of Native Antagonistic Rhizobacteria From the Peruvian Coastal Ecosystems Associated with Phaseolus vulgaris. Curr Microbiol 78, 1418–1431 (2021). https://doi.org/10.1007/s00284-021-02388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02388-x