Abstract
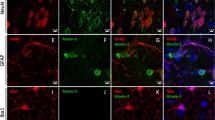
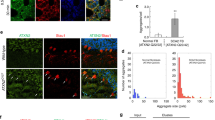
There is still no treatment for polyglutamine disorders, but clearance of mutant proteins might represent a potential therapeutic strategy. Autophagy, the major pathway for organelle and protein turnover, has been implicated in these diseases. To determine whether the autophagy/lysosome system contributes to the pathogenesis of spinocerebellar ataxia type 7 (SCA7), caused by expansion of a polyglutamine tract in the ataxin-7 protein, we looked for biochemical, histological and transcriptomic abnormalities in components of the autophagy/lysosome pathway in a knock-in mouse model of the disease, postmortem brain and peripheral blood mononuclear cells (PBMC) from patients. In the mouse model, mutant ataxin-7 accumulated in inclusions immunoreactive for the autophagy-associated proteins mTOR, beclin-1, p62 and ubiquitin. Atypical accumulations of the autophagosome/lysosome markers LC3, LAMP-1, LAMP2 and cathepsin-D were also found in the cerebellum of the SCA7 knock-in mice. In patients, abnormal accumulations of autophagy markers were detected in the cerebellum and cerebral cortex of patients, but not in the striatum that is spared in SCA7, suggesting that autophagy might be impaired by the selective accumulation of mutant ataxin-7. In vitro studies demonstrated that the autophagic flux was impaired in cells overexpressing full-length mutant ataxin-7. Interestingly, the expression of the early autophagy-associated gene ATG12 was increased in PBMC from SCA7 patients in correlation with disease severity. These results provide evidence that the autophagy/lysosome pathway is impaired in neurons undergoing degeneration in SCA7. Autophagy/lysosome-associated molecules might, therefore, be useful markers for monitoring the effects of potential therapeutic approaches using modulators of autophagy in SCA7 and other autophagy/lysosome-associated neurodegenerative disorders.








Similar content being viewed by others
Abbreviations
- SCA7:
-
Spinocerebellar ataxia type 7
- polyQ:
-
Polyglutamine
- ATXN7:
-
Ataxin-7
- KI:
-
Knock-in
- PBMC:
-
Peripheral blood mononuclear cells
- PC:
-
Purkinje cell
- ATG:
-
Autophagy-related protein
- LC3:
-
Microtubule-associated protein 1 light chain (MAP1) light chain 3
- Lamp-1:
-
Lysosomal-associated membrane protein 1
- Lamp-2:
-
Lysosomal associated membrane protein 2
- PML:
-
Promyelocytic leukemia protein
- APP:
-
Amyloid precursor protein
References
Benton CS, de Silva R, Rutledge SL, Bohlega S, Ashizawa T, Zoghbi HY (1998) Molecular and clinical studies in SCA-7 define a broad clinical spectrum and the infantile phenotype. Neurology 51:1081–1086
Cancel G, Duyckaerts C, Holmberg M, Zander C, Yvert G, Lebre AS, Ruberg M et al (2000) Distribution of ataxin-7 in normal human brain and retina. Brain 123(Pt 12):2519–2530
Chort A, Alves S, Marinello M, Dufresnois B, Dornbierer JG, Tesson C, Latouche M et al (2013) Interferon beta induces clearance of mutant ataxin 7 and improves locomotion in SCA7 knock-in mice. Brain 136:1732–1745. doi:10.1093/brain/awt061
Chou AH, Chen CY, Chen SY, Chen WJ, Chen YL, Weng YS, Wang HL (2010) Polyglutamine-expanded ataxin-7 causes cerebellar dysfunction by inducing transcriptional dysregulation. Neurochem Int 56:329–339. doi:10.1016/j.neuint.2009.11.003
Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L et al (2010) Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE 5:e9313. doi:10.1371/journal.pone.0009313
Cuervo AM, Wong E (2014) Chaperone-mediated autophagy: roles in disease and aging. Cell Res 24:92–104. doi:10.1038/cr.2013.153
David G, Abbas N, Stevanin G, Durr A, Yvert G, Cancel G, Weber C et al (1997) Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet 17:65–70
Eskelinen EL (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502. doi:10.1016/j.mam.2006.08.005
Gusella JF, MacDonald ME (2000) Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat Rev Neurosci 1:109–115
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M et al (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889. doi:10.1038/nature04724
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. doi:10.1146/annurev-genet-102808-114910
Helmlinger D, Hardy S, Sasorith S, Klein F, Robert F, Weber C, Miguet L et al (2004) Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum Mol Genet 13:1257–1265
Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, Osmand A et al (2010) Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet 19:3702–3720. doi:10.1093/hmg/ddq285
Holmberg M, Duyckaerts C, Durr A, Cancel G, Gourfinkel-An I, Damier P, Faucheux B et al (1998) Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum Mol Genet 7:913–918
Ichimura Y, Komatsu M (2010) Selective degradation of p62 by autophagy. Semin Immunopathol 32:431–436. doi:10.1007/s00281-010-0220-1
Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E et al (2008) Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem 283:22847–22857. doi:10.1074/jbc.M802182200
Janer A, Martin E, Muriel MP, Latouche M, Fujigasaki H, Ruberg M, Brice A et al (2006) PML clastosomes prevent nuclear accumulation of mutant ataxin-7 and other polyglutamine proteins. J Cell Biol 174:65–76
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728. doi:10.1093/emboj/19.21.5720
Kegel KB, Kim M, Sapp E, McIntyre C, Castano JG, Aronin N, DiFiglia M (2000) Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci 20:7268–7278
Klionsky DJ, Codogno P, Cuervo AM, Deretic V, Elazar Z, Fueyo-Margareto J, Gewirtz DA et al (2010) A comprehensive glossary of autophagy-related molecules and processes. Autophagy 6:438–448. doi:10.4161/auto.6.4.12244
Levine B, Klionsky DJ (2004) Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 6:463–477 pii: S1534580704000991
Ma JF, Huang Y, Chen SD, Halliday G (2010) Immunohistochemical evidence for macroautophagy in neurones and endothelial cells in Alzheimer’s disease. Neuropathol Appl Neurobiol 36:312–319. doi:10.1111/j.1365-2990.2010.01067.x
Martinez-Vicente M, Talloczy Z, Wong E, Tang G, Koga H, Kaushik S, de Vries R et al (2010) Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nat Neurosci 13:567–576. doi:10.1038/nn.2528
Michalik A, Martin JJ, Van Broeckhoven C (2004) Spinocerebellar ataxia type 7 associated with pigmentary retinal dystrophy. Eur J Hum Genet 12:2–15. doi:10.1038/sj.ejhg.52011085201108
Mizushima N (2010) The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol 22:132–139. doi:10.1016/j.ceb.2009.12.004
Mizushima N, Noda T, Ohsumi Y (1999) Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J 18:3888–3896. doi:10.1093/emboj/18.14.3888
Mookerjee S, Papanikolaou T, Guyenet SJ, Sampath V, Lin A, Vitelli C, DeGiacomo F et al (2009) Posttranslational modification of ataxin-7 at lysine 257 prevents autophagy-mediated turnover of an N-terminal caspase-7 cleavage fragment. J Neurosci 29:15134–15144. doi:10.1523/JNEUROSCI.4720-09.2009
Nakamura Y, Tagawa K, Oka T, Sasabe T, Ito H, Shiwaku H, La Spada AR et al (2012) Ataxin-7 associates with microtubules and stabilizes the cytoskeletal network. Hum Mol Genet 21:1099–1110. doi:10.1093/hmg/ddr539
Nascimento-Ferreira I, Nobrega C, Vasconcelos-Ferreira A, Onofre I, Albuquerque D, Aveleira C, Hirai H et al (2013) Beclin 1 mitigates motor and neuropathological deficits in genetic mouse models of Machado-Joseph disease. Brain 136:2173–2188. doi:10.1093/brain/awt144
Nascimento-Ferreira I, Santos-Ferreira T, Sousa-Ferreira L, Auregan G, Onofre I, Alves S, Dufour N et al (2011) Overexpression of the autophagic beclin-1 protein clears mutant ataxin-3 and alleviates Machado-Joseph disease. Brain 134:1400–1415. doi:10.1093/brain/awr047
Palhan VB, Chen S, Peng GH, Tjernberg A, Gamper AM, Fan Y, Chait BT et al (2005) Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc Natl Acad Sci USA 102:8472–8477
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A et al (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282:24131–24145. doi:10.1074/jbc.M702824200
Pankiv S, Lamark T, Bruun JA, Overvatn A, Bjorkoy G, Johansen T (2010) Nucleocytoplasmic shuttling of p62/SQSTM1 and its role in recruitment of nuclear polyubiquitinated proteins to promyelocytic leukemia bodies. J Biol Chem 285:5941–5953. doi:10.1074/jbc.M109.039925
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S et al (2008) The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest 118:2190–2199. doi:10.1172/JCI33585
Ramachandran N, Munteanu I, Wang P, Ruggieri A, Rilstone JJ, Israelian N, Naranian T et al (2013) VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy. Acta Neuropathol 125:439–457. doi:10.1007/s00401-012-1073-6
Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O’Kane CJ, Brown SD et al (2005) Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet 37:771–776. doi:10.1038/ng1591
Ravikumar B, Duden R, Rubinsztein DC (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum Mol Genet 11:1107–1117
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F et al (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595. doi:10.1038/ng1362ng1362
Rosenfeldt MT, Nixon C, Liu E, Mah LY, Ryan KM (2012) Analysis of macroautophagy by immunohistochemistry. Autophagy 8:963–969. doi:10.4161/auto.20186
Rub U, Schols L, Paulson H, Auburger G, Kermer P, Jen JC, Seidel K et al (2013) Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Prog Neurobiol 104:38–66. doi:10.1016/j.pneurobio.2013.01.001
Sarkar S, Krishna G, Imarisio S, Saiki S, O’Kane CJ, Rubinsztein DC (2008) A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum Mol Genet 17:170–178. doi:10.1093/hmg/ddm294
Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA et al (2007) Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol 3:331–338. doi:10.1038/nchembio883
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol 124:1–21. doi:10.1007/s00401-012-1000-x
Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, MacDonald M et al (2006) Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem 281:14474–14485. doi:10.1074/jbc.M600364200
Shvets E, Fass E, Scherz-Shouval R, Elazar Z (2008) The N-terminus and Phe52 residue of LC3 recruit p62/SQSTM1 into autophagosomes. J Cell Sci 121:2685–2695. doi:10.1242/jcs.026005
Sittler A, Walter S, Wedemeyer N, Hasenbank R, Scherzinger E, Eickhoff H, Bates GP et al (1998) SH3GL3 associates with the Huntingtin exon 1 protein and promotes the formation of polygln-containing protein aggregates. Mol Cell 2:427–436
Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A et al (2009) Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci 29:13578–13588. doi:10.1523/JNEUROSCI.4390-09.2009
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A et al (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS ONE 5:e9979. doi:10.1371/journal.pone.0009979
Stevanin G, Durr A, Brice A (2000) Clinical and molecular advances in autosomal dominant cerebellar ataxias: from genotype to phenotype and physiopathology. Eur J Hum Genet 8:4–18. doi:10.1038/sj.ejhg.5200403
Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R et al (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science 307:1282–1288. doi:10.1126/science.1105681
Tanaka M, Machida Y, Niu S, Ikeda T, Jana NR, Doi H, Kurosawa M et al (2004) Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat Med 10:148–154
van de Warrenburg BP, Notermans NC, Schelhaas HJ, van Alfen N, Sinke RJ, Knoers NV, Zwarts MJ et al (2004) Peripheral nerve involvement in spinocerebellar ataxias. Arch Neurol 61:257–261. doi:10.1001/archneur.61.2.25761/2/257
Wong E, Cuervo AM (2010) Autophagy gone awry in neurodegenerative diseases. Nat Neurosci 13:805–811. doi:10.1038/nn.2575
Wong E, Cuervo AM (2010) Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol 2:a006734. doi:10.1101/cshperspect.a006734
Wu G, Wang X, Feng X, Zhang A, Li J, Gu K, Huang J et al (2011) Altered expression of autophagic genes in the peripheral leukocytes of patients with sporadic Parkinson’s disease. Brain Res 1394:105–111. doi:10.1016/j.brainres.2011.04.013
Yoo SY, Pennesi ME, Weeber EJ, Xu B, Atkinson R, Chen S, Armstrong DL et al (2003) SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron 37:383–401
Yu X, Ajayi A, Boga NR, Strom AL (2012) Differential degradation of full-length and cleaved ataxin-7 fragments in a novel stable inducible SCA7 model. J Mol Neurosci 47:219–233. doi:10.1007/s12031-012-9722-8
Yue Z, Holstein GR, Chait BT, Wang QJ (2009) Using genetic mouse models to study the biology and pathology of autophagy in the central nervous system. Methods Enzymol 453:159–180. doi:10.1016/S0076-6879(08)04008-1
Acknowledgments
This study was supported by grants from the French National Research Agency (ANR-07-MRAR-025-01 to A.S), the French Association against Myopathies (AFM, to AB and long-term fellowship to SA), the French association Connaitre les Syndrômes Cérébelleux (short-term fellowship to SA), and the French Foundation for Medical Research (FRM, to JCC and FC-D), as well as the “Investissements d’avenir” program ANR-10-IAIHU-06 (to the Brain and Spine Institute, Paris). We thank Prof. H. Zoghbi (Baylor College of Medicine, Houston, Texas, USA) for the SCA7 KI mice. We are grateful to Pr. C. Duyckaerts for brain samples and Drs. C. Marelli and C. Jauffret for blood samples from SCA7 patients. We would also like to thank the Cellular Imaging Platform of the Pitié Salpêtrière, especially Dr. A. Dauphin, for advice on confocal imaging, and J. Garrigue and W. Carpentier for technical assistance. The authors have no additional financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
F. Cormier-Dequaire and M. Marinello contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alves, S., Cormier-Dequaire, F., Marinello, M. et al. The autophagy/lysosome pathway is impaired in SCA7 patients and SCA7 knock-in mice. Acta Neuropathol 128, 705–722 (2014). https://doi.org/10.1007/s00401-014-1289-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1289-8