Abstract
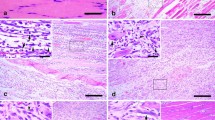
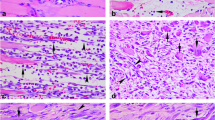
The study on time-dependent expression of α7 nicotine acetylcholine receptor (α7nAChR) was performed by immunohistochemistry, Western blotting, and real-time PCR during skeletal muscle wound healing in rats. Furthermore, co-localization of α7nAChR with macrophage or myofibroblast marker was detected by double immunofluorescence. A total of 50 Sprague–Dawley male rats were divided into control and contusion groups (3 h, 6 h, 12 h, 1 day, 3 days, 5 days, 7 days, 10 days, and 14 days post-injury). In the uninjured controls, α7nAChR positive staining was observed in the sarcolemma and sarcoplasm of normal myofibers. In wounded specimens, a small number of polymorphonuclear cells, a number of macrophages and myofibroblasts showed positive reaction for α7nAChR in contused zones. Morphometrically, the average ratios of α7nAChR-positive cells were over 50 % from 3 to 10 days after contusion, and exceeded 60 % at 5 and 7 days post-injury. Besides, the positive ratios of α7nAChR were <50 % at the other posttraumatic intervals. By Western blotting analysis, the average ratio of α7nAChR protein expression maximized at 7 days after injury, which was >2.13. Similarly, the relative quantity of α7nAChR mRNA expression peaked at 7 days post-wounding as compared with control by real-time PCR detection, showing a relative quantity of >2.65. In conclusion, the expression of α7nAChR is upregulated and temporally distributed in macrophages and myofibroblasts during skeletal muscle wound healing, which might be closely involved in inflammatory response and fibrotic repair after injury. Moreover, α7nAChR is promising as a useful marker for wound age determination of skeletal muscle.






Similar content being viewed by others
References
Ishida Y, Kimura A, Nosaka M, Kuninaka Y, Takayasu T, Eisenmenger W, Kondo T (2012) Immunohistochemical analysis on cyclooxygenase-2 for wound age determination. Int J Legal Med 126:435–440
Takamiya M, Saigusa K, Kumagai R, Nakayashiki N, Aoki Y (2005) Studies on mRNA expression of tissue-type plasminogen activator in bruises for wound age estimation. Int J Leg Med 119:16–21
Sato Y, Ohshima T (2000) The expression of mRNA of proinflammatory cytokines during skin wound healing in mice: a preliminary study for forensic wound age estimation (II). Int J Leg Med 113:140–145
Kondo T, Ohshima T, Mori R, Guan DW, Ohshima K, Eisenmenger W (2002) Immunohistochemical detection of chemokines in human skin wounds and its application to wound age determination. Int J Legal Med 116:87–91
Hayashi T, Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2004) Forensic application of VEGF expression to skin wound age determination. Int J Legal Med 118:320–325
Kagawa S, Matsuo A, Yagi Y, Ikematsu K, Tsuda R, Nakasono I (2009) The time-course analysis of gene expression during wound healing in mouse skin. Leg Med 11:70–75
Zhao R, Guan DW, Zhang W, Du Y, Xiong CY, Zhu BL, Zhang JJ (2009) Increased expressions and activations of apoptosis-related factors in cell signaling during incised skin wound healing in mice: a preliminary study for forensic wound age estimation. Leg Med 11:S155–S160
Sun JH, Wang YY, Zhang L, Gao CR, Zhang LZ, Guo Z (2010) Time-dependent expression of skeletal muscle troponin I mRNA in the contused skeletal muscle of rats: a possible marker for wound age estimation. Int J Legal Med 124:27–33
Yu TS, Cheng ZH, Li LQ, Zhao R, Fan YY, Du Y, Ma WX, Guan DW (2010) The cannabinoid receptor type 2 is time-dependently expressed during skeletal muscle wound healing in rats. Int J Legal Med 124:397–404
Prisk V, Huard J (2003) Muscle injuries and repair: the role of prostaglandins and inflammation. Histol Histopathol 18:1243–1256
Charge SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Kurzen H, Wessler I, Kirkpatrick CJ, Kawashima K, Grando SA (2007) The non-neuronal cholinergic system of human skin. Horm Metab Res 39:125–135
Karlin A (2002) Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci 3:102–114
Xiu J, Nordberg A, Zhang JT, Guan ZZ (2005) Expression of nicotinic receptors on primary cultures of rat astrocytes and upregulation of the alpha7, alpha4 and beta2 subunits in response to nanomolar concentrations of the beta-amyloid peptide (1-42). Neurochem Int 47:281–290
Kummer W, Lips KS, Pfeil U (2008) The epithelial cholinergic system of the airways. Histochem Cell Biol 130:219–234
Liu RH, Mizuta M, Matsukura S (2004) The expression and functional role of nicotinic acetylcholine receptors in rat adipocytes. J Pharmacol Exp Ther 310:52–58
Metz CN, Tracey KJ (2005) It takes nerve to dampen inflammation. Nat Immunol 6:756–757
Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA (2007) Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol 37:186–192
Fan YY, Yu TS, Wang T, Liu WW, Zhao R, Zhang ST, Ma WX, Zheng JL, Guan DW (2011) Nicotinic acetylcholine receptor α7 subunit is time-dependently expressed in distinct cell types during skin wound healing in mice. Histochem Cell Biol 135:375–387
Zhang ST, Zhao R, Ma WX, Fan YY, Guan WZ, Wang J, Ren P, Zhong K, Yu TS, Pi JB, Guan DW (2013) Nrf1 is time-dependently expressed and distributed in the distinct cell types after trauma to skeletal muscles in rats. Histol Histopathol 28:725–735
Fan YY, Ye GH, Lin KZ, Yu LS, Wu SZ, Dong MW, Han JG, Feng XP, Li XB (2013) Time-dependent expression and distribution of Egr-1 during skeletal muscle wound healing in rats. J Mol Histol 44:75–81
Kawashima K, Fujii T (2008) Basic and clinical aspects of non-neuronal acetylcholine: overview of non-neuronal cholinergic systems and their biological significance. J Pharmacol Sci 106:167–173
Kawashima K, Fujii T (2003) The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci 74:675–696
Tracey KJ (2002) The inflammatory reflex. Nature 420:853–859
Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421:384–388
Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER (2002) Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung. Association with alpha7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 26:31–41
Rehan VK, Wang Y, Sugano S, Romero S, Chen X, Santos J, Khazanchi A, Torday JS (2005) Mechanism of nicotine-induced pulmonary fibroblast transdifferentiation. Am J Physiol Lung Cell Mol Physiol 289:L667–L676
Betz P (1994) Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int J Legal Med 107:60–68
Kondo T, Tanaka J, Ishida Y, Mori R, Takayasu T, Ohshima T (2002) Ubiquitin expression in skin wounds and its application to forensic wound age determination. Int J Legal Med 116:267–272
Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2008) Expression of oxygen-regulated protein 150 (ORP150) in skin wound healing and its application for wound age determination. Int J Legal Med 122:409–414
Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2009) Detection of fibrocytes in human skin wounds and its application for wound age determination. Int J Legal Med 123:299–304
Kondo T, Ohshima T, Eisenmenger W (1999) Immunohistochemical and morphometrical study on the temporal expression of interleukin-1α (IL-1α) in human skin wounds for forensic wound age determination. Int J Legal Med 112:249–252
Betz P (1995) Immunohistochemical parameters for the age estimation of human skin wounds. A review. Am J Forensic Med Pathol 16:203–209
Bai R, Wan L, Shi M (2008) The time-dependent expressions of IL-1beta, COX-2, MCP-1 mRNA in skin wounds of rabbits. Forensic Sci Int 175:193–197
Zheng JL, Yu TS, Li XN, Fan YY, Ma WX, Du Y, Zhao R, Guan DW (2012) Cannabinoid receptor type 2 is time-dependently expressed during skin wound healing in mice. Int J Legal Med 126:807–814
Ma WX, Yu TS, Fan YY, Zhang ST, Ren P, Wang SB, Zhao R, Pi JB, Guan DW (2011) Time-dependent expression and distribution of monoacylglycerol lipase during the skin-incised wound healing in mice. Int J Legal Med 125:549–558
Cecchi R (2010) Estimating wound age: looking into the future. Int J Legal Med 124:523–536
Kondo T (2007) Timing of skin wounds. Leg Med (Tokyo) 9:109–114
Ohshima T (2000) Forensic wound examination. Forensic Sci Int 113:153–164
Hernández-Cueto C, Girela E, Sweet DJ (2000) Advances in the diagnosis of wound vitality: a review. Am J Forensic Med Pathol 21:21–31
Oehmichen M (2004) Vitality and time course of wounds. Forensic Sci Int 144:221–231
Ohshima T, Sato Y (1998) Time-dependent expression of interleukin-10 (IL-10) mRNA during the early phase of skin wound healing as a possible indicator of wound vitality. Int J Leg Med 111:251–255
Acknowledgment
This study was financially supported in part by grants from research funds for Zhejiang Provincial Natural Science Foundation of China (Q13H150005) and National Natural Science Foundation of China (81301640).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, YY., Zhang, ST., Yu, LS. et al. The time-dependent expression of α7nAChR during skeletal muscle wound healing in rats. Int J Legal Med 128, 779–786 (2014). https://doi.org/10.1007/s00414-014-1001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-014-1001-5