Abstract
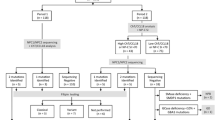
Late-onset Niemann-Pick type C (NP-C) is a rare, underdiagnosed lysosomal disease with neurological manifestations. A specific treatment, miglustat, can stabilize the disease if given early. Recently, three plasma screening biomarkers (PSBs) were developed [cholestane3β,5α,6βtriol (C-triol), 7-ketocholesterol (7-KC), and lysosphingomyelin-509 (LSM-509)], allowing a simpler and quite robust screening of patients suitable for genetic testing. The objective of our study was to evaluate practical utility and feasibility of large-scale PSB screening for NP-C in selected adult patients. Patients were prospectively enrolled if they showed, starting from 12 years of age, at least one of the three initial neuro-psychiatric manifestations described in NP-C: (1) gait disorder (cerebellar and/or dystonic); (2) cognitive decline with frontal lobe syndrome; (3) atypical psychosis. PSBs were measured in plasma of all patients and, if positive (LSM-509 and/or C-triol + 7-KC elevated), sequencing of NPC1 and NPC2 genes was performed. A total of 251 patients [136 males, 115 females; median age 42.1 (range 12.2–85.6) years] were screened. Six patients had positive PSBs. Two were confirmed to have NP-C (0.8% diagnostic yield, both with all three PSBs highly increased, especially LSM-509). False-positive rate was 1.2%, which was identical if only considering LSM-509. By contrast, false-positive rates were 8.1% and 5.7% for 7-KC and C-triol, respectively. We showed that selecting patients with neurologic and/or psychiatric symptoms consistent with NP-C for large-scale PSB screening is a simple and valid strategy to identify new adult NP-C patients, and would probably lead to earlier diagnosis and treatment administration if widely applied.




Similar content being viewed by others
References
Nadjar Y et al (2018) Adult Niemann-Pick disease type C in France: clinical phenotypes and long-term miglustat treatment effect. Orphanet J Rare Dis 13(1):175
Vanier MT (2010) Niemann-Pick disease type C. Orphanet J Rare Dis 5(1):16
Pineda M, Walterfang M, Patterson MC (2018) Miglustat in Niemann-Pick disease type C patients: a review. Orphanet J Rare Dis 13(1):1–21
Romanello M et al (2016) Comprehensive evaluation of plasma 7-ketocholesterol and cholestan-3β,5α,6β-triol in an italian cohort of patients affected by Niemann-Pick disease due to NPC1 and SMPD1 mutations. Clin Chim Acta 455(3):39–45
Giese AK et al (2015) A novel, highly sensitive and specific biomarker for Niemann-Pick type C1 disease. Orphanet J Rare Dis 10(1):1–8
Deodato F et al (2018) The impact of biomarkers analysis in the diagnosis of Niemann-Pick C disease and acid sphingomyelinase deficiency. Clin Chim Acta 486(August):387–394
Wijburg FA et al (2012) Development of a Suspicion Index to aid diagnosis of Niemann-Pick disease type C. Neurology 78(20):1560–1567
Bauer P et al (2013) Genetic screening for Niemann-Pick disease type C in adults with neurological and psychiatric symptoms: findings from the ZOOM study. Hum Mol Genet 22(21):4349–4356
Schicks J et al (2013) Niemann-pick type C is frequent in adult ataxia with cognitive decline and vertical gaze palsy. Neurology 80(12):1169–1170
Ribas GS et al (2016) Selective screening of Niemann-Pick type C Brazilian patients by cholestane-3β,5α,6β-triol and chitotriosidase measurements followed by filipin staining and NPC1/NPC2 gene analysis. Clin Chim Acta 459:57–62
Reunert J et al (2016) Rapid diagnosis of 83 patients with Niemann Pick type C disease and related cholesterol transport disorders by cholestantriol screening. EBioMedicine 4:170–175
Hammerschmidt TG et al (2018) Molecular and biochemical biomarkers for diagnosis and therapy monitorization of Niemann-Pick type C patients. Int J Dev Neurosci 66:18–23
Zhang H et al (2014) Diagnosis of Niemann-Pick disease type C with 7-ketocholesterol screening followed by NPC1/NPC2 gene mutation confirmation in Chinese patients. Orphanet J Rare Dis 9(1):1–10
Cozma C et al (2018) Lyso-SM-509 as highly sensitive biomarker for Niemann-Pick disease types A/B and C: three years experience. Mol Genet Metab 123(2):S34
Porter FD et al (2010) Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med 2(56):56ra81–56ra81
Pajares S et al (2015) Cholestane-3β,5α,6β-triol: high levels in Niemann-Pick type C, cerebrotendinous xanthomatosis, and lysosomal acid lipase deficiency. J Lipid Res 56(10):1926–1935
Pettazzoni M et al (2017) LC-MS/MS multiplex analysis of lysosphingolipids in plasma and amniotic fluid: a novel tool for the screening of sphingolipidoses and Niemann-Pick type C disease. PLoS ONE 12(7):1–19
Acknowledgements
We thank Actelion pharmaceutical and Claudia Bertonati for the financial support of this study.
Author information
Authors and Affiliations
Contributions
DM contributed to acquisition and analysis of data and drafting of the most part of manuscript and figures. MP, ILB, CE, AM, GC, AC, AD, AA, CM, MA, CT, NM, CRJ, JL, MS, LH, PE, SB, CG, DW, CP, MP, SB, and FL contributed to acquisition and analysis of data. YN contributed to conception and design of the study, acquisition and analysis of data, and drafting a significant portion of manuscript and figures.
Corresponding author
Ethics declarations
Conflicts of interest
Dr Marion Plaze, Mathieu Anheim, Cécile Pagan, Cyril Goizet, and Yann Nadjar received travel grants and/or honoraria from Actelion Pharmaceuticals, which manufactures the drug Miglustat, used in NP-C; Mathieu Anheim also received honoraria and/or grants from Johnson and Johnson which is now part of Actelion Pharmaceuticals. Dr Daniele Mandia, Caroline Moreau, Isabelle Le Ber, Claire Ewenczyk, Alexandre Morin, Guilhem Carle, Angèle Consoli, Adrian Degardin, Ali Amad, Mathieu Anheim, Christine Tranchant, Nicolas Mélé, Carole Roue Jagot, Julien Lagarde, Marie Sarazin, Lorraine Hamelin, Pierre Ellul, Soumeya Bekri, Serge Belliard, David Wallon, and Foudil Lamari report no conflicts of interests relevant to this manuscript.
Ethical standards
This study has been approved by the appropriate ethics committee.
Informed consent
All patients gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Mandia, D., Plaze, M., Le Ber, I. et al. High diagnostic value of plasma Niemann-Pick type C biomarkers in adults with selected neurological and/or psychiatric disorders. J Neurol 267, 3371–3377 (2020). https://doi.org/10.1007/s00415-020-10020-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10020-4