Abstract
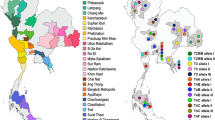
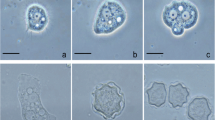
Vermamoeba vermiformis represents one of the most common free-living amoebae identified in worldwide environmental surveys. We analyzed 56 water samples with varying characteristics, including temperature and the particular settings in which humans may be exposed to water, plus one corneal scraping from a keratitis patient, with the following aims: (i) to investigate the presence of V. vermiformis; (ii) to identify the isolate subtypes; (iii) to place the Italian isolates in the broader picture of the genetic diversity within V. vermiformis. Twenty-two isolates were identified upon culturing and sequencing of > 600 bp in the 18S ribosomal RNA (rRNA) gene sequence, bringing to 27 the number of sequences recovered from Italian sources. By adding deposited sequences, we assembled a dataset of 74 isolates. Three of our isolates were characterized by allelic code 7-5-1-1, never reported before, and two showed 100% identity with an uncultured eukaryote and carried the 719T>C variant. We show that the variable segments E5, E3, F, and G convey most of the information on diversity, enabling the clustering of the isolates in a replicable fashion. The presence of different strains in natural thermal waters and in distribution systems indicated heterogeneity of the amoebic populations. Also, ours and the only other sequence from human infection were mapped in different clades. Overall, we enlarged the repertoire of single nucleotide and indel variants and the list of allelic codes, proceeding one step further in the description of the diversity within the genus.

Similar content being viewed by others
References
Aitken D, Hay J, Kinnear FB, Kirkness CM, Lee WR, Seal DV (1996) Amebic keratitis in a wearer of disposable contact lenses due to a mixed Vahlkampfia and Hartmannella infection. Ophthalmology 103:485–494
Cavalier-Smith T, Chao EE, Lewis R (2016) 187-gene phylogeny of protozoan phylum Amoebozoa reveals a new class (Cutosea) of deep-branching, ultrastructurally unique, enveloped marine Lobosa and clarifies amoeba evolution. Mol Phylogenet Evol 99:275–296
Centeno M, Rivera F, Cerva L, Tsutsumi V, Gallegos E, Calderón A, Ortiz R, Bonilla P, Ramirez E, Suarez G (1996) Hartmannella vermiformis isolated from the cerebrospinal fluid of a young male patient with meningoencephalitis and bronchopneumonia. Arch Med Res 27:579–586
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500
Delafont V, Rodier MH, Maisonneuve E, Cateau E (2018) Vermamoeba vermiformis: a free-living amoeba of interest. Microb Ecol 76:991–1001. https://doi.org/10.1007/s00248-018-1199-8
Gunderson JH, Goss SJ, Sogin ML (1994) The sequence of the Hartmannella vermiformis small subunit rRNA coding region. J Eukaryot Microbiol 41:481–482
Inoue T, Asari S, Tahara K, Hayashi K, Kiritoshi A, Shimomura Y (1998) Acanthamoeba keratitis with symbiosis of Hartmannella ameba. Am J Ophthalmol 125:721–723
Kang S, Tice AK, Spiegel FW, Silberman JD, Pánek T, Cepicka I, Kostka M, Kosakyan A, Alcântara DMC, Roger AJ, Shadwick LL, Smirnov A, Kudryavtsev A, Lahr DJG, Brown MW (2017) Between a pod and a hard test: the deep evolution of amoebae. Mol Biol Evol 34:2258–2270
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kuiper MW, Valster RM, Wullings BA, Boonstra H, Smidt H, van der Kooij D (2006) Quantitative detection of the free-living amoeba Hartmannella vermiformis in surface water by using real-time PCR. Appl Environ Microbiol 72:5750–5756
Lorenzo-Morales J, Martínez-Carretero E, Batista N, Alvarez-Marín J, Bahaya Y, Walochnik J, Valladares B (2007) Early diagnosis of amoebic keratitis due to a mixed infection with Acanthamoeba and Hartmannella. Parasitol Res 102:167–169
McGinnis S, Madden TL (2004) BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res 32:W20–W25
Montalbano Di Filippo M, Santoro M, Lovreglio P, Monno R, Capolongo C, Calia C, Fumarola L, D’Alfonso R, Berrilli F, Di Cave D (2015) Isolation and molecular characterization of free-living amoebae from different water sources in Italy. Int J Environ Res Public Health 12:3417–3427
Montalbano Di Filippo M, Novelletto A, Di Cave D, Berrilli F (2017) Identification and phylogenetic position of Naegleria spp. from geothermal springs in Italy. Exp Parasitol 183:143–149
Niyyati M, Rahimi F, Lasejerdi Z, Rezaeian M (2014) Potentially pathogenic free−living amoebae in contact lenses of the asymptomatic contact lens wearers. Iranian J Parasitol 9:14–19
Smirnov A, Nassonova E, Berney C, Fahrni J, Bolivar I, Pawlowski J (2005) Molecular phylogeny and classification of the lobose amoebae. Protist 156:129–142
Smirnov AV, Chao E, Nassonova ES, Cavalier-Smith T (2011) A revised classification of naked lobose amoebae (Amoebozoa: lobosa). Protist 162:545–570
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tsvetkova N, Schild M, Panaiotov S, Kurdova-Mintcheva R, Gottstein B, Walochnik J, Aspock H, Lucas MS, Muller N (2004) The identification of free-living environmental isolates of amoebae from Bulgaria. Parasitol Res 92:405–413
Wheeler WC (1996) Optimization alignment: the end of multiple sequence alignment in phylogenetics. Cladistics 12:1–9
Wheeler WC (2001) Homology and the optimization of DNA sequence data. Cladistics 17:S3–S11
Wheeler WC, Aagesen L, Arango CP, Faivovich J, Grant T, D’Haese C, Janies D, Smith WL, Varón A, Giribet G (2005) Dynamic homology and phylogenetic systematics: a unified approach using POY. American Museum of Natural History, New York
Funding
This study was partially funded by the University of Rome Tor Vergata, Italy (Ricerca Scientifica di Ateneo 2016 “Mission: Sustainability”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable standards.
Additional information
Handling Editor: Julia Walochnik
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
The implicit alignment corresponding to Fig. 1 in Fasta format (74 Vermamoeba sequences, outgrouped with 3 Echinamoeba sequences). Heatmap of the pairwise distances matrix produced in Excel environment. Pairwise distance matrix was generated from aligned sequences (74 Vermamoeba sequences) in MEGA v5, with equal weights for transitions and transversions. (XLSX 21 kb)
ESM 1
(PDF 387 kb)
Video 1
(FAS 126 kb)
Rights and permissions
About this article
Cite this article
Montalbano Di Filippo, M., Berrilli, F., Di Cave, D. et al. Novel data from Italian Vermamoeba vermiformis isolates from multiple sources add to genetic diversity within the genus. Parasitol Res 118, 1751–1759 (2019). https://doi.org/10.1007/s00436-019-06294-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06294-x