Abstract
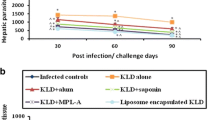
The treatment against visceral leishmaniasis (VL) presents problems, mainly related to the toxicity and/or high cost of the drugs. In this context, a prophylactic vaccination is urgently required. In the present study, a Leishmania protein called LiHyE, which was suggested recently as an antigenic marker for canine and human VL, was evaluated regarding its immunogenicity and protective efficacy in BALB/c mice against Leishmania infantum infection. In addition, the protein was used to stimulate peripheral blood mononuclear cells (PBMCs) from VL patients before and after treatment, as well as from healthy subjects. Vaccination results showed that the recombinant (rLiHyE) protein associated with liposome or saponin induced effective protection in the mice, since significant reductions in the parasite load in spleen, liver, draining lymph nodes, and bone marrow were found. The parasitological protection was associated with Th1-type cell response, since high IFN-γ, IL-12, and GM-CSF levels, in addition to low IL-4 and IL-10 production, were found. Liposome induced a better parasitological and immunological protection than did saponin. Experiments using PBMCs showed rLiHyE-stimulated lymphoproliferation in treated patients’ and healthy subjects’ cells, as well as high IFN-γ levels in the cell supernatant. In conclusion, rLiHyE could be considered for future studies as a vaccine candidate against VL.









Similar content being viewed by others
References
Abbehusen MMC, Cunha J, Suarez MS, Teixeira C, Almeida VDA, Pereira LDS, Bordoni M, Gil-Santana L, Solcà MDS, Fraga DBM, Fischer L, Bozza PT, Veras PST, Valenzuela JG, Kamhawi S, Andrade BB, Brodskyn CI (2018) Immunization of experimental dogs with salivary proteins from Lutzomyia longipalpis, using dna and recombinant canarypox virus induces immune responses consistent with protection against Leishmania infantum. Front Immunol 9:2558
Abeijon C, Daifalla N, Krautz-Peterson G, Pizzirani S, Beamer G, Frazatti-Gallina NM, Raw I, Campos-Neto A (2016) Immunogenicity in dogs and protection against visceral leishmaniasis induced by a 14 kDa Leishmania infantum recombinant polypeptide. Trials Vaccinol 5:1–7
Adem E, Tajebe F, Getahun M, Kiflie A, Diro E, Hailu A, Shkedy Z, Mengesha B, Mulaw T, Atnafu S, Deressa T, Mathewos B, Abate E, Modolell M, Munder M, Müller I, Takele Y, Kropf P (2016) Successful treatment of human visceral leishmaniasis restores antigen specific IFN-γ, but not IL-10 production. PLoS Negl Trop Dis 10:e0004468
Agallou M, Athanasiou E, Samiotaki M, Panayotou G, Karagouni E (2016) Identification of immunoreactive Leishmania infantum protein antigens to asymptomatic dog sera through combined immunoproteomics and bioinformatics analysis. PLoS One 11:e0149894
Agallou M, Pantazi E, Tsiftsaki E, Toubanaki DK, Gaitanaki C, Smirlis D, Karagouni E (2018) Induction of protective cellular immune responses against experimental visceral leishmaniasis mediated by dendritic cells pulsed with the N-terminal domain of Leishmania infantum elongation factor-2 and CpG oligodeoxynucleotides. Mol Immunol 103:7–20
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Leishmaniasis Control Team WHO (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7:e35671
Amit A, Vijayamahantesh DMR, Singh AK, Kumar V, Suman SS, Singh A, Kumar A, Thakur AK, Das VR, Das P, Bimal S (2017) Immunization with Leishmania donovani protein disulfide isomerase DNA construct induces Th1 and Th17 dependent immune response and protection against experimental visceral leishmaniasis in BALB/c mice. Mol Immunol 82:104–113
Askarizadeh A, Jaafari MR, Khamesipour A, Badiee A (2017) Liposomal adjuvant development for leishmaniasis vaccines. Ther Adv Vaccines 5:85–101
Badiee A, Khamesipour A, Samiei A, Soroush D, Shargh VH, Kheiri MT, Barkhordari F, Robert Mc Master W, Mahboudi F, Jaafari MR (2012) The role of liposome size on the type of immune response induced in BALB/c mice against leishmaniasis: rgp63 as a model antigen. Exp Parasitol 132:403–409
Badiee A, Heravi Shargh V, Khamesipour A, Jaafari MR (2013) Micro/nanoparticle adjuvants for antileishmanial vaccines: present and future trends. Vaccine 31:735–749
Bogdan C (2008) Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell Microbiol 10:1221–1234
Botana L, Matía B, San Martin JV, Romero-Maté A, Castro A, Molina L, Fernandez L, Ibarra-Meneses A, Aguado M, Sánchez C, Horrillo L, Chicharro C, Nieto J, Ortega S, Ruiz-Giardin JM, Carrillo E, Moreno J (2018) Cellular markers of active disease and cure in different forms of Leishmania infantum-induced disease. Front Cell Infect Microbiol 8:381
Brito RCF, Cardoso JMO, Reis LES, Mathias FAS, Aguiar-Soares RDO, Teixeira-Carvalho A, Roatt BM, Corrêa-Oliveira R, Ruiz JC, Resende DM, Reis AB (2019) Synthetic peptides elicit strong cellular immunity in visceral leishmaniasis natural reservoir and contribute to long-lasting polyfunctional T-cells in BALB/c mice. Vaccines (Basel) 7:e162
Caldas A, Favali C, Aquino D, Vinhas V, Van Weyenbergh J, Brodskyn C, Costa J, Barral-Netto M, Barral A (2005) Balance of IL-10 and interferon-gamma plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BMC Infect Dis 5:113
Cillari E, Vitale G, Arcoleo F, D’Agostino P, Mocciaro C, Gambino G, Malta R, Stassi G, Giordano C, Milano S, Mansueto S (1995) In vivo and in vitro cytokine profiles and mononuclear cell subsets in Sicilian patients with active visceral leishmaniasis. Cytokine 7:740–745
Coelho EAF, Tavares CAP, Carvalho FAA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71:3988–3994
Coelho VT, Oliveira JS, Valadares DG, Chávez-Fumagalli MA, Duarte MC, Lage PS, Soto M, Santoro MM, Tavares CA, Fernandes AP, Coelho EA (2012) Identification of proteins in promastigote and amastigote-like Leishmania using an immunoproteomic approach. PLoS Negl Trop Dis 6:e1430
Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, Rolf T, Misquith A, Laurance JD, Raman VS, Bailor HR, Cauwelaert ND, Reed SJ, Vallur A, Favila M, Orr MT, Ashman J, Ghosh P, Mondal D, Reed SG (2015) From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunology 4:e35
Costa LE, Goulart LR, Pereira NC, Lima MI, Duarte MC, Martins VT, Lage PS, Menezes-Souza D, Ribeiro TG, Melo MN, Fernandes AP, Soto M, Tavares CA, Chávez-Fumagalli MA, Coelho EA (2014) Mimotope-based vaccines of Leishmania infantum antigens and their protective efficacy against visceral leishmaniasis. PLoS One 9:e110014
Costa LE, Alves PT, Carneiro AP, Dias ACS, Fujimura PT, Araujo GR, Tavares GSV, Ramos FF, Duarte MC, Menezes-Souza D, Briza P, Briza FF, Coelho EAF, Goulart LR (2019) Leishmania infantum β-tubulin identified by reverse engineering technology through phage display applied as theranostic marker for human visceral leishmaniasis. Int J Mol Sci 20:e1812
Das P, Paik D, Naskar K, Chakraborti T (2018) Leishmania donovani serine protease encapsulated in liposome elicits protective immunity in experimental visceral leishmaniasis. Microbes Infect 20:37–47
Dayakar A, Chandrasekaran S, Kuchipudi SV, Kalangi SK (2019) Cytokines: key determinants of resistance or disease progression in visceral leishmaniasis: opportunities for novel diagnostics and immunotherapy. Front Immunol 10:670
Dias DS, Ribeiro PAF, Martins VT, Lage DP, Portela ÁSB, Costa LE, Salles BCS, Lima MP, Ramos FF, Santos TTO, Caligiorne RB, Chávez-Fumagalli MA, Silveira JAG, Magalhães-Soares DF, Gonçalves DU, Oliveira JS, Roatt BM, Duarte MC, Menezes-Souza D, Silva ES, Galdino AS, Machado-de-Ávila RA, Teixeira AL, Coelho EAF (2017) Recombinant small glutamine-rich tetratricopeptide repeat-containing protein of Leishmania infantum: potential vaccine and diagnostic application against visceral leishmaniasis. Mol Immunol 91:272–281
Dias DS, Ribeiro PAF, Martins VT, Lage DP, Costa LE, Chávez-Fumagalli MA, Ramos FF, Santos TTO, Ludolf F, Oliveira JS, Mendes TAO, Silva ES, Galdino AS, Duarte MC, Roatt BM, Menezes-Souza D, Teixeira AL, Coelho EAF (2018a) Vaccination with a CD4+ and CD8+ T-cell epitopes-based recombinant chimeric protein derived from Leishmania infantum proteins confers protective immunity against visceral leishmaniasis. Transl Res 200:18–34
Dias DS, Martins VT, Ribeiro PAF, Ramos FF, Lage DP, Tavares GSV, Mendonça DVC, Chávez-Fumagalli MA, Oliveira JS, Silva ES, Gomes DA, Rodrigues MA, Duarte MC, Galdino AS, Menezes-Souza D, Coelho EAF (2018b) Antigenicity, immunogenicity and protective efficacy of a conserved Leishmania hypothetical protein against visceral leishmaniasis. Parasitology 145:740–751
Duarte MC, Pimenta DC, Menezes-Souza D, Magalhães RD, Diniz JL, Costa LE, Chávez-Fumagalli MA, Lage PS, Bartholomeu DC, Alves MJ, Fernandes AP, Soto M, Tavares CA, Gonçalves DU, Rocha MO, Coelho EA (2015) Proteins selected in Leishmania (Viannia) braziliensis by an immunoproteomic approach with potential serodiagnosis applications for tegumentary leishmaniasis. Clin Vaccine Immunol 22:1187–1196
Duarte MC, Lage DP, Martins VT, Chávez-Fumagalli MA, Roatt BM, Menezes-Souza D, Goulart LR, Soto M, Tavares CA, Coelho EA (2016) Recent updates and perspectives on approaches for the development of vaccines against visceral leishmaniasis. Rev Soc Bras Med Trop 49:398–407
Gannavaram S, Dey R, Avishek K, Selvapandiyan A, Salotra P, Nakhasi HL (2014) Biomarkers of safety and immune protection for genetically modified live attenuated Leishmania vaccines against visceral leishmaniasis - discovery and implications. Front Immunol 5:241
Gatto M, Abreu MM, Tasca KI, de Assis GM, da Silva LD, Simão JC, Fortaleza CM, de Campos Soares ÂM, Calvi SA (2015) The involvement of TLR2 and TLR4 in cytokine and nitric oxide production in visceral leishmaniasis patients before and after treatment with anti-leishmanial drugs. PLoS One 10:e0117977
Ghalib HW, Piuvezam MR, Skeiky YA, Siddig M, Hashim FA, El-Hassan AM, Russo DM, Reed SG (1993) Interleukin-10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest 92:324–329
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Grenfell RF, Marques-da-Silva EA, Souza-Testasicca MC, Coelho EA, Fernandes AP, Afonso LC, Rezende AS (2010) Antigenic extracts of Leishmania braziliensis and Leishmania amazonensis associated with saponin partially protects BALB/c mice against Leishmania chagasi infection by suppressing IL-10 and IL-4 production. Mem Inst Oswaldo Cruz 105:818–822
Grimaldi G Jr, Teva A, Dos-Santos CB, Santos FN, Pinto ID, Fux B, Leite GR, Falqueto A (2017) Field trial of efficacy of the Leish-Tec® vaccine against canine leishmaniasis caused by Leishmania infantum in an endemic area with high transmission rates. PLoS One 12:e0185438
Gupta SK, Sisodia BS, Sinha S, Hajela K, Naik S, Shasany AK, Dube A (2007) Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics 7:816–823
Henriksen-Lacey M, Korsholm KS, Andersen P, Perrie Y, Christensen D (2011) Liposomal vaccine delivery systems. Expert Opin Drug Deliv 8:505–519
Ibarra-Meneses AV, Ghosh P, Hossain F, Chowdhury R, Mondal D, Alvar J, Moreno J, Carrillo E (2017) IFN-γ, IL-2, IP-10, and MIG as biomarkers of exposure to Leishmania spp., and of cure in human visceral leishmaniasis. Front cell infect Microbiol 7:200
Joshi S, Yadav NK, Rawat K, Kumar V, Ali R, Sahasrabuddhe AA, Siddiqi MI, Haq W, Sundar S, Dube A (2019) Immunogenicity and protective efficacy of T-cell epitopes derived from potential Th1 stimulatory proteins of Leishmania (Leishmania) donovani. Front Immunol 10:288
Kaur H, Thakur A, Kaur S (2015) Studies on cocktails of 31-kDa, 36-kDa and 51-kDa antigens of Leishmania donovani along with saponin against murine visceral leishmaniasis. Parasite Immunol 37:192–203
Kedzierski L, Evans KJ (2014) Immune responses during cutaneous and visceral leishmaniasis. Parasitology 30:1–19
Keerti YNK, Joshi S, Ratnapriya S, Sahasrabuddhe AA, Dube A (2018) Immunotherapeutic potential of Leishmania (Leishmania) donovani Th1 stimulatory proteins against experimental visceral leishmaniasis. Vaccine 36:2293–2299
Koutsoni OS, Barhoumi M, Guizani I, Dotsika E (2019) New insights on the adjuvant properties of the Leishmania infantum eukaryotic initiation factor. J Immunol Res 2019:9124326
Kumar R, Singh N, Gautam S, Singh OP, Gidwani K, Rai M, Sacks D, Sundar S, Nylén S (2014) Leishmania specific CD4 T cells release IFN-γ that limits parasite replication in patients with visceral leishmaniasis. PLoS Negl Trop Dis 8:e3198
Lage DP, Martins VT, Duarte MC, Garde E, Chávez-Fumagalli MA, Menezes-Souza D, Roatt BM, Tavares CA, Soto M, Coelho EA (2015) Prophylactic properties of a Leishmania-specific hypothetical protein in a murine model of visceral leishmaniasis. Parasite Immunol 37:646–656
Lage DP, Machado AS, Ramos FF, Silveira PC, Dias DS, Ribeiro PAF, Tavares GSV, Costa LE, Santos TTO, Steiner BT, Fagundes MI, Chávez-Fumagalli MA, Lyon S, Moreira RLF, Duarte MC, Menezes-Souza D, Caligiorne RB, Machado-de-Ávila RA, Teixeira AL, Coelho EAF (2019) A biomarker for tegumentary and visceral leishmaniasis based on a recombinant Leishmania hypothetical protein. Immunobiology 224:477–484
Lima BS, Fialho LC Jr, Pires SF, Tafuri WL, Andrade HM (2016) Immunoproteomic and bioinformatic approaches to identify secreted Leishmania amazonensis, L. braziliensis, and L. infantum proteins with specific reactivity using canine serum. Vet Parasitol 223:115–119
Margaroni M, Agallou M, Athanasiou E, Kammona O, Kiparissides C, Gaitanaki C, Karagouni E (2017) Vaccination with poly(D,L-lactide-co-glycolide) nanoparticles loaded with soluble Leishmania antigens and modified with a TNFα-mimicking peptide or monophosphoryl lipid A confers protection against experimental visceral leishmaniasis. Int J Nanomedicine 12:6169–6184
Martins VT, Chávez-Fumagalli MA, Costa LE, Canavaci AM, Martins AM, Lage PS, Lage DP, Duarte MC, Valadares DG, Magalhães RD, Ribeiro TG, Nagem RA, Darocha WD, Régis WC, Soto M, Coelho EA, Fernandes AP, Tavares CA (2013) Antigenicity and protective efficacy of a Leishmania amastigote-specific protein, member of the super-oxygenase family, against visceral leishmaniasis. PLoS Negl Trop Dis 7:e2148
Martins VT, Duarte MC, Lage DP, Costa LE, Carvalho AM, Mendes TA, Roatt BM, Menezes-Souza D, Soto M, Coelho EA (2017) A recombinant chimeric protein composed of human and mice-specific CD4+ and CD8+ T-cell epitopes protects against visceral leishmaniasis. Parasite Immunol 39:e12359
Noormehr H, Zavaran Hosseini A, Soudi S, Beyzay F (2018) Enhancement of Th1 immune response against Leishmania cysteine peptidase A, B by PLGA nanoparticle. Int Immunopharmacol 59:97–105
O’Hagan DT, Gregorio E (2009) The path to a successful vaccine adjuvant: "the long and winding road". Drug Discov Today 14:541–551
Peruhype-Magalhães V, Martins-Filho OA, Prata A, Silva LA, Rabello A, Teixeira-Carvalho A, Figueiredo RM, Guimarães-Carvalho SF, Ferrari TC, Correa-Oliveira R (2005) Immune response in human visceral leishmaniasis: analysis of the correlation between innate immunity cytokine profile and disease outcome. Scand J Immunol 62:487–495
Petrovsky N, Aguilar JC (2004) Vaccine adjuvants: current state and future trends. Immunol Cell Biol 82:488–496
Pirdel L, Farajnia S (2017) a non-pathogenic recombinant Leishmania expressing lipophosphoglycan 3 against experimental infection with Leishmania infantum. Scand J Immunol 86:15–22
Portela ÁSB, Costa LE, Salles BCS, Lima MP, Santos TTO, Ramos FF, Lage DP, Martins VT, Caligiorne RB, Lessa DR, Silva FR, Machado AS, Nascimento GF, Gama IS, Chávez-Fumagalli MA, Teixeira AL, Rocha MOC, Rocha RL, Coelho EAF (2018) Identification of immune biomarkers related to disease progression and treatment efficacy in human visceral leishmaniasis. Immunobiology 223:303–309
Rajput ZI, Hu SH, Xiao CW, Arijo AG (2007) Adjuvant effects of saponins on animal immune responses. J Zhejiang Univ Sci B 8:153–161
Ramakrishnan A, Schumack NM, Gariepy CL, Eggleston H, Nunez G, Espinoza N, Nieto M, Castillo R, Rojas J, McCoy AJ, Beck Z, Matyas GR, Alving CR, Guerry P, Poly F, Laird RM (2019) Enhanced immunogenicity and protective efficacy of a Campylobacter jejuni conjugate vaccine coadministered with liposomes containing monophosphoryl lipid a and QS-21. mSphere 4:e00101
Raman VS, Duthie MS, Fox CB, Matlashewski G, Reed SG (2012) Adjuvants for Leishmania vaccines: from models to clinical application. Front Immunol 3:144
Ramos FF, Costa LE, Dias DS, Santos TTO, Rodrigues MR, Lage DP, Salles BCS, Martins VT, Ribeiro PAF, Chávez-Fumagalli MA, Dias ACS, Alves PT, Vieira ÉLM, Roatt BM, Menezes-Souza D, Duarte MC, Teixeira AL, Goulart LR, Coelho EAF (2017) Selection strategy of phage-displayed immunogens based on an in vitro evaluation of the Th1 response of PBMCs and their potential use as a vaccine against Leishmania infantum infection. Parasit Vectors 10:617
Rao M, Onkar S, Peachman KK, White Y, Trinh HV, Jobe O, Zhou Y, Dawson P, Eller MA, Matyas GR, Alving CR (2018) Liposome-encapsulated human immunodeficiency virus-1 gp120 induces potent V1V2-specific antibodies in humans. J Infect Dis 218:1541–1550
Ratnapriya S, Keerti SAA, Dube A (2019) Visceral leishmaniasis: an overview of vaccine adjuvants and their applications. Vaccine 37:3505–3519
Rey-Ladino J, Ross AG, Cripps AW, McManus DP, Quinn R (2011) Natural products and the search for novel vaccine adjuvants. Vaccine 29:6464–6471
Ribeiro PAF, Dias DS, Novais MVM, Lage DP, Tavares GSV, Mendonça DVC, Oliveira JS, Chávez-Fumagalli MA, Roatt BM, Duarte MC, Menezes-Souza D, Ludolf F, Tavares CAP, Oliveira MC, Coelho EAF (2018a) A Leishmania hypothetical protein-containing liposome-based formulation is highly immunogenic and induces protection against visceral leishmaniasis. Cytokine 111:131–139
Ribeiro PAF, Dias DS, Lage DP, Costa LE, Salles BCS, Steiner BT, Ramos FF, Lima MP, Santos TTO, Chaves AT, Chávez-Fumagalli MA, Fujiwara RT, Bueno LL, Caligiorne RB, Magalhães-Soares DF, Silveira JAG, Machado-de-Ávila RA, Gonçalves DU, Coelho EAF (2018b) A conserved Leishmania hypothetical protein evaluated for the serodiagnosis of canine and human visceral and tegumentary leishmaniasis, as well as a serological marker for the posttreatment patient follow-up. Diagn Microbiol Infect Dis 92:196–203
Ribeiro PAF, Dias DS, Lage DP, Martins VT, Costa LE, Santos TTO, Ramos FF, Tavares GSV, DVC M, Ludolf F, Gomes DA, Rodrigues MA, Chávez-Fumagalli MA, Silva ES, Galdino AS, Duarte MC, Roatt BM, Menezes-Souza D, Teixeira AL, Coelho EAF (2019) Immunogenicity and protective efficacy of a new Leishmania hypothetical protein applied as a DNA vaccine or in a recombinant form against Leishmania infantum infection. Mol Immunol 106:108–118
Rijal S, Ostyn B, Uranw S, Rai K, Bhattarai NR, Dorlo T, Beijnen JH, Vanaerschot M, Decuypere S, Dhakal SS, Das ML, Karki P, Singh R, Boelaert M, Dujardin JC (2013) Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin Infect Dis 56:1530–1538
Sabur A, Asad M, Ali N (2016) Lipid based delivery and immuno-stimulatory systems: master tools to combat leishmaniasis. Cell Immunol 309:55–60
Sabur A, Bhowmick S, Chhajer R, Ejazi SA, Didwania N, Asad M, Bhattacharyya A, Sinha U, Ali N (2018) Lipossomal elongation factor-1α triggers effector CD4 and CD8 T cells for induction of long-lasting protective immunity against visceral leishmaniasis. Front Immunol 9:18
Santos TTO, Martins VT, Lage DP, Costa LE, Salles BCS, Carvalho AMRS, Dias DS, Ribeiro PAF, Chávez-Fumagalli MA, Machado-de-Ávila RA, Roatt BM, de Magalhães-Soares DF, Menezes-Souza D, Coelho EAF, Duarte MC (2017) Probing the efficacy of a heterologous Leishmania Viannia braziliensis recombinant enolase as a candidate vaccine to restrict the development of L. infantum in BALB/c mice. Acta Trop 171:8–16
Schaut RG, Grinnage-Pulley TL, Esch KJ, Toepp AJ, Duthie MS, Howard RF, Reed SG, Petersen CA (2016) Recovery of antigen-specific T cell responses from dogs infected with Leishmania (L.) infantum by use of vaccine associated TLR-agonist adjuvant. Vaccine 34:5225–5234
Singh OP, Stober CB, Singh AK, Blackwell JM, Sundar S (2012) Cytokine responses to novel antigens in an Indian population living in an area endemic for visceral leishmaniasis. PLoS Negl Trop Dis 6:e1874
Singh N, Sundar S (2017) Inflammatory chemokines and their receptors in human visceral leishmaniasis: gene expression profile in peripheral blood, splenic cellular sources and their impact on trafficking of inflammatory cells. Mol Immunol 85:111–119
Souza MA, Castro MC, Oliveira AP, Almeida AF, Almeida TM, Reis LC, Medeiros ÂC, Brito ME, Pereira VR (2013) Cytokines and NO in American tegumentary leishmaniasis patients: profiles in active disease, after therapy and in self-healed individuals. Microb Pathog 57:27–32
Sundar S, Singh A (2016) Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther Adv Infect Dis 3:98–109
Thakur A, Kaur H, Kaur S (2015) Studies on the protective efficacy of freeze thawed promastigote antigen of Leishmania donovani along with various adjuvants against visceral leishmaniasis infection in mice. Immunobiology 220:1031–1038
Vijayamahantesh AA, Dikhit MR, Singh AK, Venkateshwaran T, Das VNR, Das P, Bimal S (2017) Immuno-informatics based approaches to identify CD8+ T cell epitopes within the Leishmania donovani 3-ectonucleotidase in cured visceral leishmaniasis subjects. Microbes Infect 19:358–369
World Health Organization. (2018) Leishmaniasis. http://www.who.int/topics/leishmaniasis/en/
Wowk PF, Franco LH, Fonseca DMD, Paula MO, Vianna ÉDSO, Wendling AP, Augusto VM, Elói-Santos SM, Teixeira-Carvalho A, Silva FDC, Vinhas SA, Martins-Filho OA, Palaci M, Silva CL, Bonato VLD (2017) Mycobacterial Hsp65 antigen upregulates the cellular immune response of healthy individuals compared with tuberculosis patients. Hum Vaccin Immunother 13:1040–1050
Ye J, Yang Y, Dong W, Gao Y, Meng Y, Wang H, Li L, Jin J, Ji M, Xia X, Chen X, Jin Y, Liu Y (2019) Drug-free mannosylated liposomes inhibit tumor growth by promoting the polarization of tumor-associated macrophages. Int J Nanomedicine 14:3203–3220
Acknowledgements
The authors thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ (Belo Horizonte, Minas Gerais, Brazil) for use of its facilities. The authors would like thank to CAPES, CNPq, and FAPEMIG for the scholarships. This work was supported by grants from CNPq (APQ-408675/2018-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Sarah Hendrickx
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ribeiro, P.A.F., Dias, D.S., Lage, D.P. et al. Evaluation of the protective efficacy of a Leishmania protein associated with distinct adjuvants against visceral leishmaniasis and in vitro immunogenicity in human cells. Parasitol Res 119, 2609–2622 (2020). https://doi.org/10.1007/s00436-020-06752-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-020-06752-x