Abstract
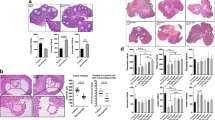
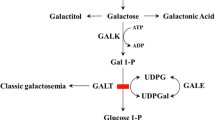
Galactosemia is a genetic disease with deficiency of galactose-1-uridyltransferase, resulting in the accumulation of galactose or galactose-1-phosphate in the blood and tissues. Rats were fed with normal rat chow and with a high-galactose diet for 4 weeks to give control and galactosemic groups, and their ovarian function was studied. The two groups of rats were injected with pregnant mare's serum gonadotrophin (PMSG) and were killed at different time points after human chorionic gonadotrophin (hCG) injection. The number of oocytes ovulated in the controls was significantly higher than in the galactosemic group. Morphometric studies of the ovaries also showed a higher number of corpora lutea in the controls. Western blot analysis of granulosa cells showed that the overall expressions of Fas and FasL were lower in the control group and their expressions of inhibitor of apoptosis proteins (IAPs) were higher than in the galactosemic group, especially at 8 h post hCG injection. TDT-mediated dUTP-biotin nick end-labeling (TUNEL) and immunohistochemical staining of ovarian sections with Ki-67 and IAPs showed more apoptotic granulosa cells in the galactosemic group and the expressions of IAPs in granulosa cells also confirmed the result of the Western blot. These findings support our hypothesis that ovarian dysfunction in galactosemic rats is due to increased apoptosis in granulosa cells of maturing follicles.




Similar content being viewed by others
References
Adashi EY, Resnick CE, D'Ercole AJ, Svoboda ME, Van-Wyk JJ (1985) Insulin-like growth factors as intraovarian regulators of granulosa cell growth and function. Endocr Rev 6:400–420
Ambrosini G, Adida C, Altieri DC (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 3:917–921
Bank N, Coco M, Aynedjian HS (1989) Galactose feeding causes glomerular hyper-perfusion: prevention by aldose reductase inhibition. Am J Physiol 256:F994–F999
Beutler E (1991) Galactosemia: screening and diagnosis. Clin Biochem 24:293–300
Billig H, Furuta I, Hsueh AJW (1994) Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis. Endocrinology 133:2204–2212
Bomsel-Helmreich O, Gougeon A, Thebault A, Saltarelli D, Milgrom E, Frydman R, Papiernik E (1979) Healthy and atretic human follicles in the preovulatory phase: differences in evolution of follicular morphology and steroid content of follicular fluid. J Clin Endocrinol Metab 48:686–694
Byskov AGS (1979) Atresia. In: Midgley AR, Sadler WA (eds) Ovarian follicular development and function. Raven, New York, pp 41–57
Chun SY, Eisenhauer KM, Kubo M, Hsueh AJW (1995) Interleukin-1β suppresses apoptosis in rat ovarian follicles by increasing nitric oxide production. Endocrinology 136:3120–3127
Chung MA (1997) Galactosemia in infancy: diagnosis, management and prognosis. Pediatr Nurs 23:563–569
Daniels BS, Hauser EB (1992) Glycation of albumin, not glomerular basement membrane alters permeability in an in vitro model. Diabetes 41:1415–1421
Deveraux QL, Takahashi R, Salvesen GS, Reed JC (1997) X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388:300–304
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 18:5242–5251
Erickson GF, Magoffin DA, Dyer CA, Hofeditz C (1985) The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev 6:371–399
Fraser IS, Russell P, Greco S, Robertson DM (1986) Resistant ovary syndrome and premature ovarian failure in young women with galactosemia. Clin Reprod Fertil 4:133–138
Gabbay KH, O'Sullivan JB (1968) The sorbitol pathway in diabetes and galactosemia: enzyme and substrate localization and changes in kidney. Diabetes 17:300
Ghiggeri GM, Candiano G, Delfino G, Queirolo C (1985) Electrical charge of serum and urinary albumin in normal and diabetic humans. Kidney Int 28:168–177
Gitzelmann R (1969) Estimation of galactose-1-phosphate in erythrocytes: a rapid and simple enzymatic method. Clin Chim Acta 26:313–316
Gitzelmann R (1995) Galactose-1-phosphate in pathophysiology of galactosemia. Eur J Pediatr 154 (Suppl 2):s45–s49
Gitzelmann R, Curtius HC, Schneller I (1967) Galactitol and galactose-1-phosphate in the lens of galactosemic infant. Exp Eye Res 6:1–3
Gorospe WC, Hughes FMJ, Spangelo BL (1992) Interleukin-6: effects on and production by rat granulosa cells in vitro. Endocrinology 130:1750–1752
Greenwald GS (1989) Temporal and topographic changes in DNA synthesis after induced follicular atresia. Biol Reprod 41:175–181
Gundersen HJG, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–262
Hakuno N, Koji T, Yano T, Kobayashi N, Tsutsumi O, Taketani Y, Nakane PK (1996) Fas/APO-1/CD 95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 137:1938–1948
Harman SM, Louvet JP, Ross GT (1975) Interaction of estrogen and gonadotrophins on follicular atresia. Endocrinology 96:1145–1152
Himelstein-Braw R, Byskov AGS, Peters H, Faber M (1976) Follicular atresia in the infant human ovary. J Reprod Fertil 46:55–59
Hirshfield AN (1989) Rescue of atretic follicles in vitro and in vivo. Biol Reprod 40:181–190
Hirshfield AN (1991) Development of follicles in the mammalian ovary. Inv Rev Cytol 124:43–101
Hsu SY, Hsueh AJW (2000) Tissue-specific BcL-2 protein partners in apoptosis: an ovarian paradigm. Physiol Rev 80:593–614
Hsueh AJW, Jones PB (1981) Extrapituitary actions of gonadotrophin-releasing hormone. Endocr Rev 2:437–461
Kasof GM, Gomes BC (2001) Livin, a novel inhibitor of apoptosis (IAP) family member. J Biol Chem 276:3238–3246
Kaufman FR, Kogut MD, Donnell GN, Goebelsmann U, March C, Koch R (1981) Hypergonadotrophic hypo-gonadism in female patients with galactosemia. N Engl J Med 304:994–998
Kaufman FR, Xu YK, Ng WG, Donnell GN (1988) Correlation of ovarian function with galactose-1-phosphate uridyl transferase levels in galactosemia. J Pediatr 112:754–756
Kaufman FR, McBride-Chang C, Manis FR, Wolff JA, Nelson MD (1995) Cognitive functioning, neurologic status and brain imaging in classical galactosemia. Eur J Pediatr 154 (Suppl 2):S2–S5
Kim JM, Boone DL, Auyeung A, Tsang BK (1998) Granulosa cell apoptosis induced at the penultimate stage of follicular development is associated with increased levels of Fas and Fas ligand in the rat ovary. Biol Reprod 58:1170–1176
Kim JM, Yoon YD, Tsang BK (1999) Involvement of the Fas/Fas ligand system in p53-mediated granulosa cell apoptosis during follicular development and atresia. Endocrinology 140:2307–2317
Kondo H, Maruo T, Peng X, Mochizuki M (1996) Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicular regression and atresia. J Clin Endocrinol Metab 81:2702–2710
Leslie ND, Yager KL, McNamara DP, Segal S (1996) A mouse model of galactose-1-phosphate uridyl transferase deficiency. Biochem Mol Med 59:7–12
Levy HL, Driscoll SG, Porensky RS, Wener DF (1984) Ovarian failure in galactosemia. N Engl J Med 310:50
Li J, Kim JM, Liston P, Li M, Miyazaki T, Machenzie AE, Korneluk RG, Tsang BK (1998) Expression of inhibitor of apoptosis proteins (IAPs) in rat granulosa cells during ovarian follicular development and atresia. Endocrinology 139:1321–1328
Lin JH, Deng G, Huang Q, Morser J (2000) KIAP, a novel member of the inhibitor of apoptosis protein family. Biochem Biophys Res Commun 279:820–831
Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda JE, MacKenzie A, Korneluk RG (1996) Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379:349–353
Lorentz WBJ, Shibabi ZK, Weidner N (1987) Galactosemic nephropathy in the rat. Clin Physiol Biochem 5:261–267
Matsumoto K, Nakayama T, Sakai H, Tanemura K, Osuga H, Sato E, Ikeda JE (1999) Neuronal apoptosis inhibitory protein (NAIP) may enhance the survival of granulosa cells thus indirectly affecting oocyte survival. Mol Reprod Dev 54:103–111
Meyer WR, Doyle MB, Grifo JA, Lipetz KJ, Oates PJ, DeCherney AH, Diamond MP (1992) Aldose reductase inhibition prevents galactose-induced ovarian dysfunction in the Sprague-Dawley rat. Am J Obstet Gynecol 167:1837–1843
Prestoz LLC, Couto AS, Shin YS, Petry KG (1997) Altered follicle stimulating hormone isoforms in female galactosemia patients. Eur J Pediatr 156:116–120
Quirk SM, Cowan RG, Joshi SG, Henrikson KP (1995) Fas antigen-mediated apoptosis in human granulosa/luteal cells. Biol Reprod 52:279–287
Robison WGJ, Kador PF, Kinoshita JH (1983) Retinal capillaries: basement membrane thickening by galactosemia prevented with aldose reductase inhibitor. Science 221:1177–1179
Roy N, Mahadevan MS, McLean M, Shutler G, Yaraghi Z, Farahani R, Baird S, Besner-Johnston A, Lefebvre C, Kang X, Salih M, Aubry H, Tamai K, Guan X, Ioannou P, Crawford TO, Jong PJ, Surh L, Ikeda J-E, Korneluk RG, MacKenzie A (1995) The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80:167–178
Sadrkhanloo R, Hofeditz C, Erickson GF (1987) Evidence for widespread atresia in the hypophysectomized estrogen-treated rat. Endocrinology 120:146–155
Sakamaki K, Yoshida H, Nishimura Y, Nishikawa S, Manabe N, Yonehara S (1997) Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol Reprod Dev 47:11–18
Schwarz V (1960) The value of galactose phosphate determination in the treatment of galactosemia. Arch Dis Child 35:428–432
Schwarz V, Goldberg L (1955) Galactose-1-phosphate in galactose cataract. Biochim Biophys Acta 18:310–311
Schweitzer S, Shin Y, Jakobs C, Brodehl J (1993) Long-term outcome in 134 patients with galactosemia. Eur J Pediatr 152:36–43
Shin YS, Gathof BS, Podskarbi T, Sommer M, Giugliani R, Gresser U (1996) Three missense mutations in the galactose-1-phosphate uridyltransferase gene of three families with mild galactosemia. Eur J Pediatr 155:393–397
Sippel TO (1966) Changes in the water, protein and glutathione contents of the lens in the course of galactose cataract development in rats. Invest Ophthalmol 5:568–575
Swartz WJ, Mattison DR (1988) Galactose inhibition of ovulation in mice. Fertil Steril 49:522–526
Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC (1998) A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 273:7787–7790
Tsafriri A, Braw RH (1984) Experimental approaches to atresia in mammals. Oxf Rev Reprod Biol 6:226–265
Tyfield LA (2000) Galactosemia and allelic variation at the galactose-1-phosphate uridyltransferase gene: a complex relationship between genotype and phenotype. Eur J Pediatr 159(Suppl 3):s204–s207
Tyfield L, Reichardt J, Fridovich-Keil J, Croke DT, Elsas LJ 2nd, Strobl W, Kozak L, Coskun T, Novelli G, Okano Y, Zekanowski C, Shin Y, Boleda MD (1999) Classical galactosemia and mutation at the galactose-1-phosphate uridyl transferase (GALT) gene. Hum Mutat 13:417–430
Waggoner DD, Buist NRM, Donnell GN (1990) Long-term prognosis in galactosemia: results of a survey of 350 cases. J Inherit Metab Dis 13:802–818
Weibel ER, Stäubli W, Gnägi HR, Hess FA (1969) Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods and normal morphometric data for rat liver. J Cell Biol 42:68–91
Acknowledgements
We thank Dr. B.K. Tsang, Ottawa University, for his advice on this study and Ms. May Cheung and Ms. W.Y Wong for their technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This project was supported by grants from the University of Hong Kong (7289/98M, 10203016/03678/20200/302/01 and 10203720/03678/20200/323/01)
Rights and permissions
About this article
Cite this article
Lai, K.W., Cheng, L.Y.L., Cheung, A.L.M. et al. Inhibitor of apoptosis proteins and ovarian dysfunction in galactosemic rats. Cell Tissue Res 311, 417–425 (2003). https://doi.org/10.1007/s00441-002-0689-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-002-0689-6