Abstract
It is commonly presumed that plant respiratory CO2 release increases with increasing temperature. However, we report on very contrasting stem CO2 release (R S)–temperature relationships of trees in a species-rich tropical montane forest of southern Ecuador under dry and wet season conditions. Rates of R S were low and completely uncoupled from the dial temperature regime during the humid season. In contrast, during the dry season, R S was generally higher and temperature sensitivity of R S differed greatly in degree and even in the direction of response, indicating that temperature might not be the only determinant of R S. In order to explain the heterogeneity of R S, we related R S to vapour pressure deficit, wind speed and solar radiation as important abiotic drivers influencing transpiration and photosynthesis. Stepwise multiple regression analyses with these meteorological predictors either were biased by high collinearity of the independent variables or could not enhance the ability to explain the variability of R S. We assume maintenance respiration to dominate under humid conditions unfavourable for energy acquisition of the tree, thus explaining the pronounced uncoupling of R S from atmospheric parameters. In contrast, the drier and hotter climate of the dry season seems to favour R S via enhanced assimilatory substrate delivery and stem respiratory activity as well as elevated xylem sap CO2 imports with increased transpiration. In addition, tree individual differences in the temperature responses of R S may mirror diverging climatic adaptations of co-existing moist forest tree species which have their distribution centre either at higher or lower elevations.
Similar content being viewed by others
Introduction
Temperature has long been identified as the most important abiotic factor influencing plant respiratory activity due to its well known effect on enzymatic reactions (e.g. Amthor 1989). A strong relationship between temperature and plant tissue respiration has been exhaustively proved and verified under controlled conditions (e.g. Maier et al. 1998; Tjoelker et al. 1999, 2001; Atkin et al. 2000, 2006, 2007; Loveys et al. 2003; Armstrong et al. 2006). However, in the field, a consistent relationship is often not found (e.g. Edwards and McLaughlin 1978; Negisi 1982; Lavigne 1987; Gunderson et al. 2000; Teskey and McGuire 2002). Especially, woody tissue respiration is reported not to be exclusively related to temperature since other sources of CO2 than the respiratory activity of living wood cells (inner bark, cambium, xylem parenchyma) alone can influence the apparent amount of CO2 escaping through the bark. Imports of dissolved CO2 via the transpiration stream can add substantial amounts to the stem internal carbon dioxide. The quantitative contribution of the different sources to the internal flux of CO2 was found to vary considerably on a seasonal as well as daily basis. Consequently, stem internal CO2 concentrations can constantly fluctuate, which in turn can affect the amount of CO2 diffusing out of the bark (e.g. McGuire and Teskey 2004; Teskey et al. 2008; Saveyn et al. 2008a, b).
Stem respiration usually depends on substrate availability, the demand for energy equivalents and the enzymatic activity (Amthor 1995; Lambers et al. 1998; Pruyn et al. 2002, 2005; Atkin et al. 2005). However, it is still barely understood how stem CO2 release (R S) is finally controlled and how internally circulating CO2 is influencing the apparent efflux. High rates of R S coincided with high xylem sap fluxes (Levy and Jarvis 1998; Levy et al. 1999; Horna and Zimmermann 2002). Other studies reported a negative correlation between xylem flux and radial CO2 efflux (Negisi 1979; Edward and Hanson 1996; Teskey and McGuire 2002; Gansert and Burkdorf 2005; Teskey and McGuire 2007). All these studies suggested that R S might be linked to canopy water use. However, removing the foliage had little effect on the dial pattern of R S in an experiment of Maier and Clinton (2006). No relationship between sap flow and R S was found by Ceschia (2001), Carey et al. (1996) and Edwards and Wullschleger (2000).
From a biomechanical point of view, the resistance to radial gas diffusion as controlled by xylem, cambium or the bark layers (Sorz and Hietz 2006; Lendzian 2006; Steppe et al. 2007) can significantly influence the apparent stem CO2 release. Bark structural properties differed between species and these differences could explain much of the between-species variation in the velocity of oxygen diffusion in wood segments in a laboratory study by Sorz and Hietz (2006). Under controlled conditions, where abiotic influences could be excluded, Steppe et al. (2007) found a substantial variation in the resistance to radial CO2 diffusion from stem and branch wood of three Populus deltoides clones. However, Steppe et al. (2007) used branch segments from different positions within the tree canopy known to vary considerably in their pattern of CO2 release and the underlying cause of this variation remained unsolved.
Under natural conditions, where the influence of environmental factors cannot be excluded, the picture gets even more complicated due to the fact that co-occurring plants are responding individualistically to their specific environment and probably even more so to shifts in the environmental setting (Larigauderie and Körner 1995; Arnone and Körner 1997; Amthor 1989; Oren and Pataki 2001; Reich et al. 2003; Atkin et al. 2005; Kerkhoff et al. 2005; Enquist et al. 2007). A better understanding of the variability in R S among different individuals in a population, different species in a stand, and different patches of the forest is therefore crucial to predict changes in the carbon dioxide exchange between tree wood and the atmosphere, in particular, in highly diverse tropical forests (Kerkhoff et al. 2005; Enquist et al. 2007).
In this paper, we report on in situ-measurements of the diurnal rates of R S of representative tree species in a tropical montane moist forest in southern Ecuador during two hydrologically contrasting seasons of the year. Under the prevailing moist conditions, mean annual temperature is relatively low and diurnal temperature amplitudes are small. In contrast, the short dry season climate is characterised by exceptionally high day-time and low night-time temperatures. Trees of this ecosystem have to cope with constantly low temperatures, compared to lowland forests, while being exposed to unpredictable short-term microclimatic shifts. The objectives of this study were (a) to analyse the temperature-response of R S of adapted moist forest tree species during two contrasting seasons, i.e. sunny-dry versus cloudy-moist weather conditions, and (b) to compare patterns of R S of eight co-existing tree species from seven families for quantifying variability between trees. Linear regression analyses and stepwise multiple regressions with selected meteorological factors (vapour pressure deficit, wind speed, solar radiation) were used to take account of abiotic drivers controlling transpiration and photosynthesis, which may affect R S under contrasting climatic conditions. We hypothesise that plant inherent factors and external variables apart from temperature are playing an important role in determining the apparent R S at the level of individual trees.
Materials and methods
Study area and tree selection
The study was conducted in the Reserva San Francisco (RSF) adjacent to the Podocarpus National Park (PNP) on the eastern slopes of the southern Ecuadorian Andes. The study site (03°58′345′′S/79°04′648′′W) is located at 1,890 m elevation and covered by a species-rich montane forest of about 12 m in height. Forest structure and species composition are described in more detail by Röderstein et al. (2005) and Leuschner et al. (2007). The soils of the region developed from metamorphic shale, quartzite, and sandstone bedrock; they are characterised by low fertility (Schrumpf et al. 2001).
Within the stand, we selected 20 mature canopy trees representing abundant families and comprising a broad range of stem diameters. Further details on tree selection are given in Zach et al. (2008).
For the exemplary study on the impacts of the dry climate conditions on stem respiratory activity, we restricted analysis to 10 out of the 20 mature canopy trees sampled (Table 1). These trees had been measured under pronounced dry season conditions as characterised by high ambient temperature, relatively high vapour pressure deficits (D) and declining soil water availability (Fig. 1). The remaining 10 trees were measured 1 week earlier, when some rain fell, which did not allow for an appropriate comparison of wet and dry season respiratory patterns. The tree selection comprised climax species of seven families (Table 1). Most of the tree species were represented by only one individual, except Miconia punctata and Matayba inelegans of which each two individuals of similar size were sampled (Table 1). Trees were equipped with dendrometer bands for stem increment measurements. However, due to dendrometer defects, we could not determine stem increment rates for all tree individuals.
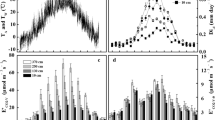
Climate data for the measurement periods in November 2005 (dry season) and April 2006 (wet season) as recorded at a nearby climate station at 2,000 m a.s.l. (M. Richter, unpublished data). Vapour pressure deficit was calculated from the climate station data (D out) and from climate data recorded inside the forest site at 2 m above ground (D in). Soil matric potential (ψS) was measured at adjacent forest sites at 2,000 m (S. Engelhard, unpublished data). Tissue temperature was calculated from air temperature with Eqs. 1 and 2
Rates of stem CO2 release (R S) of the selected tree individuals were measured during 4 days in the dry period in November 2005 (DOY 324–327; Fig. 1) and 4 days during the humid season in April 2006 (DOY 117-120). For each tree, we conducted one 24-h-course of R S with rates being logged half-hourly. The diurnal half-hour-values were averaged to calculate the mean daily rate of R S of each tree (Table 1), while the entire dataset was used for regression analysis (Table 2; Fig. 2). R S was monitored at breast height (1.3 m) using the mobile 6-chamber respiration system ANARESY 2 (Walz, Effeltrich, Germany) and an integrated LI-7000 infrared gas analyzer for CO2 and H2O (Li-Cor, Inc., Lincoln, NE, USA) running in differential mode. Details of the technical equipment are provided by Zach et al. (2008). Air temperature (T A) and relative air humidity at 2 m height inside the stand were monitored synchronously using a Rotronic sensor (Rotronic AG, Bassersdorf, Switzerland) connected to the data logger of the ANARESY system (CR 10, Campbell Scientific, Logan, UT, USA).
Rates of stem CO2 release (RS) of 10 tree individuals at 1,890 m elevation plotted against tissue temperature. Tissue temperature was calculated from air temperature with Eqs. 1 and 2. Separate regression lines are given for the dry season (November 2005, open circle) and the humid season (April 2006, filled circle) for each tree individual. Coefficients of determination are given in Table 2. For key to species’ abbreviations, see Table 1
The measured tree individuals were not equipped with thermocouple elements for stem tissue temperature (T T) monitoring. Therefore, we used data deriving from tissue temperature measurements conducted later in the year in the study site. In July 2006, thermocouples (diameter 3 mm, length 20 mm, Siemens, Munich, Germany) were installed at breast height (depth 10 mm) at two arbitrarily chosen tree stems (DBH 15 and 20 cm) for the continuous measurements for stem tissue temperature. We used data from July/August 2006 and November 2006 to establish relationships between the continuously recorded air temperature and tissue temperature:
where T A is the air temperature measured inside the stand. These equations were used to calculate tissue temperature from air temperature in our two measurement periods in 2005 where tissue temperature was not recorded directly.
Climate conditions of the study periods
In general, the climate of the study area is humid throughout the year. However, an extremely wet period (April–July) is followed by several months with less frequent rainfall (September–December) (Bendix et al. 2006). Mean annual precipitation at 1,960 m a.s.l. is ca. 2,200 mm (Emck 2007). At the study site, annual mean air temperature is 15.7°C and relative humidity 90.7% (Moser et al. 2008).
During November 2005, foehn winds caused an exceptionally dry and sunny period throughout the study area. Fires occurred frequently throughout the wider region during this period. Only 90 mm of rainfall were recorded in November, which fell during the first 2 weeks (M. Richter, unpublished data). When we started measurements in end of November, the rainless period had already lasted for 7 days. The lack of rainfall resulted in a substantial decrease of air humidity and an increase of daily temperature amplitudes compared to the wet season conditions. Vapour pressure deficit (D) continuously increased during the four consecutive measurement days in November (Fig. 1, data recorded by M. Richter at a nearby climate station, unpublished). Substantially higher wind speeds were recorded during this dry period compared to the humid days (Fig. 1). The average soil water matric potential (ψS) as measured in adjacent forest sites at 2,000 m progressively decreased during the four measurement days in November from −0.15 (±0.13) to −0.24 (±0.12) MPa at 15 cm depth and from −0.10 (±0.09) to −0.19 (±0.12) MPa at 30 cm (S. Engelhard, unpublished data). Minimum values of −0.60 MPa (15 cm) and −0.49 MPa (30 cm) were recorded at the end of the measurement period (Fig. 1).
The measurement campaign in the wet period was conducted at the beginning of the humid period in April 2006. During this month, precipitation was frequent. Low diurnal temperature amplitudes and a moderate vapour pressure deficit were characteristic for this season (Fig. 1). Data of the soil matric potential were only available for the last two measurement days. These data indicated that soil moisture exceeded field capacity with potential values greater than −0.1 MPa (S. Engelhard, unpublished data).
Data treatment
Rates of stem CO2 release (R S) were calculated as:
where R S is the CO2 release rate (μmol CO2 m−2 s−1), D [CO2] is the difference between ambient (reference gas) and chamber (sample gas) CO2 concentration, F is the molar air flow rate (mol s−1) which passes through the chamber, and AC is the surface area (m2) of the enclosed stem segment. In an earlier study (Zach et al. 2008), we found better agreement when relating the apparent CO2 efflux rates from tropical montane forest trees of South Ecuador to stem surface area than to volume.
We chose the slope of the regression curve of R S on temperature as a measure of the temperature sensitivity of R S instead of the commonly used Q 10 value. The Q 10 of respiration (i.e. the factor expressing the proportional change in respiratory CO2 release per 10°C rise in temperature) basically compares the CO2 release rate at a given temperature to that at a 10°C lower temperature (Atkin et al. 2005). Plant respiration is a process influenced by various environmental factors. Consequently, neither the Arrhenius nor the Q 10 model has been found to adequately describe the observed temperature response of respiration under field conditions. For that reason, reported measures of temperature responsiveness have to be treated with caution (Tjoelker et al. 2001; Atkin and Tjoelker 2003). In addition, there is increasing evidence that the respiratory response to temperature is neither constant nor necessarily exponential (Atkin et al. 2000, 2005; Atkin and Tjoelker 2003). Therefore, instead of focussing on few single data points on the temperature axis, we used the entire diurnal dataset of R S for analysing the temperature dependency of R S.
One important source of variation in diurnal and seasonal R S is the internal transport of dissolved CO2 in the transpiration stream influencing the apparent CO2 release through the bark (Edward and Hanson 1996; Levy and Jarvis 1998; Teskey and McGuire 2002, 2007; Gansert and Burkdorf 2005; Teskey et al. 2008). Because we did not measure xylem sap flow directly, we used D, wind speed and solar radiation as major determinants of transpiration in our regression analysis in the attempt to explain the large heterogeneity of R S.
We are aware of possible impacts of tissue resistance to the stem radial CO2 diffusion. However, we could not investigate the properties of biomechanical barriers to CO2 diffusion in our trees. Given the relationship between xylem flux and stem CO2 release and the good coupling found between climatic conditions and transpiration in the study area (Motzer et al. 2005), we assume that environmental conditions may superimpose mechanical effects of tissue resistance to CO2.
By means of linear regression, we analysed the temperature responsiveness of R S for each tree individual during the dry and the humid season. For each tree, rates of R S were plotted against the respective courses of T T and T A using either the diurnal dataset, or by considering only day-time or night-time values. To improve the explanatory power of the regression equation, we conducted linear regression analyses with the three additional meteorological predictor variables (D, wind speed, solar radiation). Using Pearson’s correlation coefficient, we quantified the influence of these four variables on R S by their r 2 . If more than one predictor showed significant effects, we conducted stepwise multiple regression analyses (α = 0.05 for tolerance; collinearity |ζ| < 0.6) to determine main causes of diurnal, day-time and night-time variability in R S. Subsequently, multiple regression analyses were repeatedly applied by removing factors with no significant influence (p < 0.05) until each individual tree model reached maximum likelihood (F test, p value).
Results
Stem CO2 release and its temperature responsiveness under dry and humid conditions
Mean daily R S was highly variable and ranged from 0.18 to 1.34 μmol m−2 s−1 during the dry, and between 0.07 and 0.64 μmol m−2 s−1 during the humid period (Table 1). Mean daily T T ranged between 15.7 and 16.8°C during the dry (minimum 10.1°C, maximum 19.9°C), and between 14.5 and 14.9°C during the humid season (minimum 10.8°C, maximum 17.5°C). In most cases, R S was higher during the dry period than under cooler and more humid conditions. Between September 2005 and April 2006, stem diameter increment differed among trees (Table 1).
Among regression models, we found linear regression to give the most appropriate fit for the relationship between R S and temperature for both periods (Fig. 2). The respective correlation between R S and T T or T A did not differ (not shown). Under dry season conditions, the response of R S to T T was highly divergent and showed strong discrepancies in the direction of response between different tree individuals. Moreover, the eight measured species showed contrasting patterns in their temperature response of R S. Besides the commonly expected positive relationship, R S was significantly negative correlated with temperature or completely uncoupled from changes in temperature in certain cases (Fig. 2). In the dry season, the temperature responsiveness of R S differed between day-time and night-time periods. Here, we found better correlations with data separated into day-time and night-time values than with the diurnal datasets in most of the cases. Most of the trees showed higher day-time than night-time rates of stem CO2 release parallel to higher day-time than night-time temperatures.
While most of the tree stems were more responsive to temperature during day-time hours, we found three tree species showing a higher temperature coupling during the night (Ia, Mi1, Cr; Table 2). The two trees showing significant negative temperature coupling under dry season conditions (Ge and Ia; see Fig. 2) showed also higher night-time than day-time rates of stem CO2 release.
The general temperature responsiveness of R S was unexpectedly low. For most of the tree individuals, plotting diurnal R S against T T yielded r² values of less than 0.5. In two of the ten measured tree species, T T could explain more than 70% of the variability in the diurnal R S. One tree of the species, Hyeronima moritziana (Hm) showed no significant relation to T T under dry season conditions neither during the day nor the night (Table 2).
In the humid season, R S was mostly uncoupled from temperature; only in some cases, a very weak correlation was observed (Table 2). Two trees showed significant relationships in the diurnal dataset (r 2 = 0.13 and 0.23). Two other trees were weakly, though significantly, correlated with T T during night-time only (r 2 = 0.22 and 0.23). For the remainder species, no differences in the temperature sensitivity of R S between day- and night-time were apparent in the humid season. Here, diurnal rates of stem CO2 release did not differ considerably between day and night.
Dependence of stem CO2 release on other meteorological predictors under dry and humid conditions
RS was linearly related to all four selected meteorological parameters (not shown). Nevertheless, neither D nor wind speed or solar radiation improved the explanatory power of the regressions with respect to RS variability under dry or humid conditions. Trees showing a significant relationship to TT also yielded comparable correlations to the other climatological parameters, most likely because of tight intercorrelation between the parameters. In most cases, stepwise multiple regression analysis was not applicable due to the high collinearity of the meteorological variables (ζ > 0.6). An exception was wind speed, which was generally less correlated to TT, D or solar radiation (ζ < 0.5).
Wind speed and radiation were the main determinants of the diurnal variation in R S of Graffenrieda emarginata (Ge) during the dry season, explaining half of the variability (model adj. r² = 0.497, F = 23.25, p < 0.0001); the correlation with radiation was a negative one (Table 2). Wind speed alone was the most important factor controlling the diurnal variation in R S of Matayba inelegans (Mi1) during the dry season (model adj. r² = 0.337, F = 24.45, p < 0.0001); again, the relation was a negative one. Wind speed improved the regression model by explaining further 10.2% of the diurnal variation in R S in Miconia punctata (Mp1). Together with T T, both parameters could explain 57.7% of the variability in diurnal R S in the dry period (F = 31.07, p < 0.0001). For the remaining tree species, multiple regression analysis either was not appropriate or could not improve the explanatory power of the model in comparison to a single-factor model with T T alone (Table 2).
Under humid conditions, the variability of R S could not be explained by any of the meteorological parameters. A few tree species showed significant, yet very weak, correlations to some of the predictors (Table 2). An exception was the night-time variability in R S in Matayba inelegans (Mi1), which was strong and negatively related to D (r² = 0.59).
Discussion
Sources of variation in humid season stem CO2 release
During the humid measurement period, R S was independent of T T (Fig. 2), and also seemed to be unaffected by any of the other investigated meteorological parameters (Table 2). Plant respiratory activity is thought to be determined by three processes, substrate supply, demand for respiratory products and potential enzyme capacity (Amthor 1995; Lambers et al. 1998; Atkin et al. 2005). Limited light availability due to cloud cover is known to strongly reduce net carbon gain of tropical forests on a seasonal as well as daily basis (Hollinger et al. 1994; Chen et al. 1999; Graham et al. 2003). In the humid season with frequent cloudiness and rainfall in the Ecuadorian Andes, assimilation rates must have been impeded, thus restricting carbohydrate supply. Porometric measurements conducted on mature canopy trees at nearby forest sites in the Podocarpus National Park showed that leaf transpiration and xylem sap flow of the tropical montane trees were substantially repressed during cloudy days and under high atmospheric humidity (Motzer et al. 2005). This may reduce the transport of dissolved CO2 with xylem water from the roots to the stem. However, amount and concentration of xylem sap CO2 mainly depend on the respiratory activity of the root system involved, while smaller amounts of CO2 may also be taken up with the soil water. Differences in fine root respiration among the measured tree individuals remain unknown as well as the quantitative contribution of root respiratory CO2 release to the transpiration stream, which might finally lower or raise rates of CO2 release through the bark.
Sorz and Hietz (2006) found higher stem water content under conditions of sufficient soil water supply to coincide with low internal oxygen contents suppressing rates of gas efflux through the bark. However, we found trees with higher (Ia, Mp2; Fig. 2) or very similar CO2 efflux patterns (Lo) under wet compared to the dry season conditions.
We assume that several factors have contributed to a slowed down R S and a low temperature sensitivity of R S, among which reduced substrate supply from photosynthesis, less demand for respiratory products under somewhat cooler temperatures, and a probably lower contribution of CO2 from the transpiration stream are the most likely.
Sources of variation in dry season stem CO2 release
The dry-season R S exceeded the wet-season rates in most of the measured trees (Fig. 2). Overall higher rates of R S in the dry season may in part be explained by an assumed higher photosynthetic carbon gain accompanied by a higher carbon investment under the sunnier and hotter conditions of the dry period. However, in the Ecuadorian montane forest, annual wood production was generally low (Moser et al. 2008) and distinct stem growth periodicities were not visible (Homeier 2004), implying that pronounced differences in growth related respiratory activity between dry and wet season were unlikely.
Temperature coupling was stronger during the dry season, though considerable scatter in the data was also evident (Fig. 2). Although the diurnal amplitude of T T and T A differed between the dry compared to the humid season (Fig. 1), the temperature response of R S to variations in T T and T A did not differ within periods (data not shown). Hence, thermal differences between T T and T A could not account for the observed discrepancies in the temperature response of R S between the two seasons. However, the actual T T of our tree stems could differ from the calculated T T values, since the continuous measurements of T T on the two stems used for the extrapolation started later in the year. Higher actual values of T T than the modelled ones could be one reason for the better temperature coupling of R S in the dry season.
Attempts to explain deviations from the common temperature–respiration relationship or high between-tree variations in stem CO2 release mainly focussed on four physiological phenomenon: (1) on time lags between temperature variation and CO2 release (Ryan 1990; Lavigne 1996; Stockfors and Linder 1998), (2) the significance of stem photosynthetic activity (Sprugel and Benecke 1991; Gansert 1995; Pfanz 1999; Strobel 2004), (3) the CO2 transport with xylem sap flux (Edward and Hanson 1996; Levy and Jarvis 1998; Teskey and McGuire 2002; Gansert and Burkdorf 2005) and/or (4) on differences in the diffusion resistance to gas by xylem, cambium and the bark (Sorz and Hietz 2006, Lendzian 2006, Steppe et al. 2007). In three of our trees (Cr, Ge, Hm; Table 1), the correlation coefficients between diurnal T T and R S were higher when a time lag of 1.5–2 h was considered in the analysis (data not shown). At least in two of the stems (Cr, Ge), R S might indeed show a delayed temperature response, which should be related to the bigger tree size prolonging the radial diffusion pathway of CO2 (Lavigne 1996) or a higher bark resistance to gas diffusion (Sorz and Hietz 2006; Lendzian 2006; Steppe et al. 2007). In the smaller stem, a lowered thermal conductivity could be the reason for the observed time delay (Hm) (Gries 2004). Bark photosynthetic activity as a significant factor could be excluded in our tree sample by comparative measurements of R S in shaded and unshaded chambers (data not shown).
Taking account of a possible influence of xylem sap CO2 to explain the large heterogeneity in R S, we related R S to D, wind speed ,and solar radiation. These parameters affect stomatal conductance and transpiration and thus the xylem sap flow with its assumed CO2 transport capacity. Motzer et al. (2005) measured leaf transpiration and xylem sap flow in mature trees nearby and found high stomatal sensitivities to D and strong stomatal control of sap flux in these trees. Even on short time scales (i.e. passing clouds), a sudden decline in leaf transpiration could be detected. Thus, the measurements of Motzer et al. (2005) evidenced a great diurnal and seasonal variation in sap flux rates which could well have resulted in large differences of CO2 transport in the xylem sap. This could explain why D, radiation, and wind speed indeed showed a significant effect on R S in a number of tree species, in particular in the dry season (Table 2). Xylem sap effects could also account for the high release rates despite low temperature sensitivities evident for most of the tree individuals.
Several authors have argued that reduced cell turgor in the stem of droughted trees could cause a transient reduction in the respiratory activity of the living tissue (e.g. Saveyn et al. 2007). This effect would offer an explanation for the apparent day-time depression of R S in Graffenrieda emerginata and Ilex amboroica (Ge, Ia; Fig. 2) during the dry season.
However, eight of the ten tree individuals showed higher, and not lower, rates of R S during the sunny/hot period. Although higher rates of R S could not be directly related to growth in case of the measured trees, the enhanced R S very likely indicated that the dry season climate principally favoured photosynthetic carbon gain and xylem sap flow, hence the delivery of substrate for plant respiratory activity as well as of dissolved CO2 from the transpiration stream, and thereby enhancing stem CO2 release.
Atmospheric uncoupling of stem respiration versus temporal efficiency in energy acquisition—evidence for climate-sensitive and climate-tolerant tree species?
Differences in rates of R S and temperature sensitivity of R S between the measured trees were much more pronounced in the dry than in the wet season (Table 1) indicating a diverging response of the trees to hotter weather conditions in this species-rich montane forest. The RSF forest with its high species diversity consists of a mixture of different tree functional types and includes typical climax and pioneer tree species co-occurring in the same stand (Homeier 2004). Moreover, our study site at 1,890 m may represent a melting point of tree species which usually occur predominantly at higher or lower elevations, thus representing different climatic adaptations. This assumed variety in tree physiological types may offer an additional explanation for the large differences in patterns of R S. For example, Ilex amboroica and Graffenrieda emarginata, the two individuals with a significant negative R S–temperature response, are mostly recorded above 2,000 m elevation in neotropical montane forests (http://www.mobot.org/mobot/Research/ecuador/search.shtml; Missouri Botanical Garden Database; Jorgensen and Yánez 1999) and thus are growing at their lower distribution limit in the RSF (J. Homeier, personal communication). Distribution preferences towards cooler and more humid climates at higher elevations would account for a stress-induced adverse reaction to the dry season conditions at RSF (Table 2; Fig. 2). In contrast, Clethra revoluta is most abundant between 1,500 and 3,500 m a.s.l. in Ecuador (Jorgensen and Yánez 1999). This species seemed to be well adapted to the local climate variability, if the strong positive temperature response during the dry season is used as a criterion to assess performance under varying thermal regimes (Fig. 2). Miconia punctata and Matayba inelegans are usually occurring in tropical lowland forests below 500 and 1,000 m elevation, respectively (http://www.mobot.org/mobot/Research/ecuador/search.shtml; Missouri Botanical Garden Database; Jorgensen and Yánez 1999). Thus, they are growing at their upper distribution limit in a rather cold environment in the RSF. These species from hotter environments responded to the dry season by a relatively large increase in R S with increasing temperature, probably revealing a better adaptation to warmer climates. In contrast, Ladenbergia oblongifolia, one of the more abundant tree species between 1,800 and 1,900 m a.s.l. in the RSF (Homeier 2004), is mainly recorded below 1,000 m elevation in the Neotropics (http://www.mobot.org/mobot/Research/ecuador/search.shtml; Missouri Botanical Garden Database). This tree individual showed a very weak R S response to increasing temperature (Table 2).
Conclusion
Our study reports on the substantial variability in stem CO2 release between seasons and among tree individuals in a species-rich tropical montane forest. Xylem sap flow effects, species-specific differences in respiratory activity or the sensitivity of R S to atmospheric factors could be influential. The plastic response in R S of adapted moist forest tree species to a drier climate could help to gain insights into the acclimation potential of a mega-diverse plant community to cope with the expected climate warming. In this context, tropical montane forests are of increasing relevance, since the predicted warming by 2–4 K during this century will most likely force mesic lowland taxa to migrate upwards (Mahli and Phillips 2004; Mayle et al. 2004). Understanding and monitoring of the present forest community composition and the species-specific responses to environmental changes are a crucial basis for conservation options and will provide clues for reliable future projections.
References
Amthor JS (1989) Respiration and crop productivity. Springer, New York
Amthor JS (1995) Terrestrial higher-plant response to increasing atmospheric CO2 in relation to the global carbon cycle. Glob Change Biol 1:243–274. doi:10.1111/j.1365-2486.1995.tb00025.x
Armstrong AF, Logan DC, Atkin OK (2006) On the developmental dependence of leaf respiration: responses to short- and long-term changes in growth temperature. Am J Bot 93:1633–1639. doi:10.3732/ajb.93.11.1633
Arnone JA, Körner C (1997) Temperature adaptation and acclimation potential of leaf dark respiration in two species of Ranunculus from warm and cold habitats. Arct Alp Res 29:122–125. doi:10.2307/1551842
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351. doi:10.1016/S1360-1385(03)00136-5
Atkin OK, Holly C, Ball MC (2000) Acclimation of snow gum (Eucalyptus pauciflora) leaf respiration to seasonal and diurnal variations in temperature: the importance of changes in capacity and temperature sensitivity of respiration. Plant Cell Environ 23:15–26. doi:10.1046/j.1365-3040.2000.00511.x
Atkin OK, Bruhn D, Hurry VM, Tjoelker MG (2005) The hot and the cold: unravelling the variable response of plant respiration to temperature. Funct Plant Biol 32:87–105. doi:10.1071/FP03176
Atkin OK, Scheurwater I, Pons TL (2006) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol 12:500–515. doi:10.1111/j.1365-2486.2006.01114.x
Atkin OK, Scheurwater I, Pons TL (2007) Respiration as a percentage of daily photosynthesis in whole plants is homeostatic at moderate, but not high, growth temperatures. New Phytol 174:367–380. doi:10.1111/j.1469-8137.2007.02011.x
Bendix J, Homeier J, Ortiz EC, Emck P, Breckle SW, Richter M, Beck E (2006) Seasonality of weather and tree phenology in a tropical evergreen mountain rain forest. Int J Biometeorol 50:370–384. doi:10.1007/s00484-006-0029-8
Carey EV, DeLucia EH, Ball JT (1996) Stem maintenance and construction respiration in Pinus ponderosa grown in different concentrations of atmospheric CO2. Tree Physiol 16:125–130
Ceschia E (2001) Environmental effects on spatial and seasonal variations of stem respiration in European beech and Norway spruce. PhD thesis. Swedish University of Agricultural Science, Uppsala, Sweden
Chen JM, Liu J, Cihlar J, Goulden ML (1999) Daily canopy photosynthesis model through temporal and spatial for remote sensing applications. Ecol Model 124:99–119. doi:10.1016/S0304-3800(99)00156-8
Edward NT, Hanson PJ (1996) Stem respiration in a closed-canopy oak forest. Tree Physiol 16:433–439
Edwards NT, McLaughlin SB (1978) Temperature-independent diel variations of respiration rates in Quercus alba and Liriodendron tulipifera. Oikos 31:200–206. doi:10.2307/3543563
Edwards NT, Wullschleger SD (2000) Carbon dioxide efflux rates from stems of mature Quercus prinus L. and Acer rubrum L. trees do not appear to be affected by sapflow rates. J Sustain For 10:125–131. doi:10.1300/J091v10n01_14
Emck P (2007) A climatology of South Ecuador. Unpublished PhD Thesis. Universität Erlangen, Germany
Enquist BJ, Kerkhoff AJ, Huxman TE, Economo EP (2007) Adaptive differences in plant physiology and ecosystem paradoxes: insights from metabolic scaling theory. Glob Change Biol 13:591–609. doi:10.1111/j.1365-2486.2006.01222.x
Gansert D (1995) Die Wurzel- und Sprossrespiration junger Buchen (Fagus sylvatica L.) in einem montanen Moder-Buchenwald. PhD thesis. University of Göttingen, Germany
Gansert D, Burkdorf M (2005) Effects of xylem sap flow on carbon dioxide efflux from stems of birch (Betula pendula Roth). Flora 200:444–455
Graham EA, Stephen SM, Kitajima K, Phillips NG, Wright SJ (2003) Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc Natl Acad Sci USA 100:572–576. doi:10.1073/pnas.0133045100
Gries D (2004) Ökophysiologische Grundlagen und biophysikalische Steuerung von der Kohlenstoffbilanz zweier edapisch gegensätzlicher Altbuchenbestände. Habilitation thesis, University of Göttingen, Germany
Gunderson CA, Norby RJ, Wullschläger SD (2000) Acclimation of photosynthesis and respiration to simulated climatic warming in northern and southern populations of Acer saccharum: laboratory and field evidence. Tree Physiol 20:87–96
Hollinger DY, Kelliher FM, Byers JN, Hunt JE, McSeveny TM, Weir PL (1994) Carbon dioxide exchange between an undisturbed old-growth temperate forest and the atmosphere. Ecol 75:134–150. doi:10.2307/1939390
Homeier J (2004) Tree diversity, forest structure and growth dynamic of two tropical montane rain forests in Ecuador und Costa Rica. Unpublished PhD thesis, University of Göttngen, Germany
Horna V, Zimmermann R (2002) Carbon release from stems and branches of two tree species with contrasting phenology in a seasonally flooded Amazon forest. In: Lieberei R, Bianchi H-K, Boehm V, Reisdorff C (eds) Neotropical Ecosystems, Proceedings of the German-Brazilian Workshop, Hamburg 2000. GKSS-Geesthacht, Germany, pp 579–587
Jorgensen PM, Yánez SL (eds) (1999) Catalogue of the vascular plants of Ecuador. Missouri Botanical Garden Press, St. Louis, Missouri
Kerkhoff AJ, Enquist BJ, Elser JJ, Fagan WF (2005) Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Glob Ecol Biogeogr 14:585–598. doi:10.1111/j.1466-822X.2005.00187.x
Lambers H, Chapin FS, Pons TI (1998) Plant physiological ecology. Springer-Verlag, New York
Larigauderie A, Körner C (1995) Acclimation of dark leaf respiration in alpine and lowland plant species. Ann Bot (Lond) 76:245–252. doi:10.1006/anbo.1995.1093
Lavigne MB (1987) Differences in stem respiration response to temperature between balsam fir trees in thinned and unthinned stands. Tree Physiol 3:225–233
Lavigne MB (1996) Comparing stem respiration and growth of jack pine provenances from northern and southern locations. Tree Physiol 16:847–852
Lendzian KJ (2006) Survival strategies of plants during secondary growth: barrier properties of phellems and lenticels towards water, oxygen and carbon dioxide. J Exp Bot 57:2535–2546. doi:10.1093/jxb/erl014
Leuschner C, Moser G, Bertsch C, Röderstein M, Hertel D (2007) Large elevation increase in the tree root/shoot ratio in a tropical mountain forest in Ecuador. Basic Appl Ecol 8:219–230. doi:10.1016/j.baae.2006.02.004
Levy PE, Jarvis PG (1998) Stem CO2 fluxes in two Sahelian shrub species (Guiera sengalensis and Combretum micranthum). Funct Ecol 12:107–116. doi:10.1046/j.1365-2435.1998.00156.x
Levy PE, Meir P, Allen SJ, Jarvis PG (1999) The effect of aqueous transport of CO2 in xylem sap on gas exchange of woody plants. Tree Physiol 19:53–59
Loveys BR, Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK (2003) Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast- and slow-growing plant species. Glob Change Biol 9:895–910. doi:10.1046/j.1365-2486.2003.00611.x
Mahli Y, Phillips OL (2004) Tropical forests and global atmospheric change: a synthesis. Phil Trans R Soc Lond Ser B 359:549–555. doi:10.1098/rstb.2003.1449
Maier CA, Clinton BD (2006) Relationship between stem CO2 efflux, stem sap velocity and xylem CO2 concentration in young loblolly pine trees. Plant Cell Environ 29:1471–1483. doi:10.1111/j.1365-3040.2006.01511.x
Maier TA, Zarnoch SJ, Dougherty PM (1998) Effects of temperature and tissue nitrogen on dormant season stem and branch respiration in a young loblolly pine (Pinus tadea) plantation. Tree Physiol 18:11–20
Mayle FE, Beerling DJ, Gosling WD, Bush MB (2004) Responses of Amazonian ecosystems to climatic and atmospheric carbon dioxide changes since the last glacial maximum. Phil Trans R Soc Lond Ser B 359:499–514. doi:10.1098/rstb.2003.1434
McGuire MA, Teskey RO (2004) Estimating stem respiration in trees by a mass balance approach that accounts for internal and external fluxes of CO2. Tree Physiol 24:571–578
Moser G, Roederstein M, Soethe N, Hertel D, Leuschner C (2008) Altitudinal changes in stand structure and biomass allocation of tropical mountain forests in relation to microclimate and soil chemistry. In: Beck E, Bendix J, Kottke I, Makeschin F, Mosandl R (eds) Gradients in a Tropical Mountain Ecosystem of Ecuador, ecological studies 198. Springer, Berlin, pp 229–242
Motzer T, Munz N, Küppers M, Schmitt D, Anhuf D (2005) Stomatal conductance, transpiration and sap flow of tropical montane rain forest trees in the Southern Ecuadorian Andes. Tree Physiol 25:1283–1293
Negisi K (1979) Bark respiration rate in stem segments detached from young Pinus densiflora trees in relation to velocity of artificial sap flow. J Jpn For Soc 61:88–93
Negisi K (1982) Diurnal fluctuations in the stem bark respiration in relationship to the wood temperature in standing young Pinus densiflora, Chamaecyparis obtusa and Quercus myrsinaefolia trees. J Jpn For Soc 64:315–319
Oren R, Pataki DE (2001) Transpiration in response to variation in microclimate and soil moisture in southeastern deciduous forests. Oecologia 127:549–559. doi:10.1007/s004420000622
Pfanz H (1999) Photosynthetic performance of twigs and stems of trees with and without stress. Phyt Ann Rei Bot 39:29–33
Pruyn ML, Gartner BL, Harmon ME (2002) Respiratory potential in sapwood of old versus young ponderosa pine trees in the Pacific Northwest. Tree Physiol 22:105–116
Pruyn ML, Gartner BL, Harmon ME (2005) Storage versus substrate limitation to bole respiratory potential in two coniferous tree species of contrasting sapwood width. J Exp Bot 56:2637–2649. doi:10.1093/jxb/eri257
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:S143–S164. doi:10.1086/374368
Röderstein M, Hertel D, Leuschner C (2005) Above- and below-ground litter production in three tropical mountain forests (South Ecuador). J Trop Ecol 21:483–483. doi:10.1017/S026646740500249X
Ryan MG (1990) Growth and maintenance respiration in stems of Pinus contorta and Pinus engelmannii. Can J Res 20:48–57. doi:10.1139/x90-008
Saveyn A, Steppe K, Lemeur R (2007) Daytime depression in tree stem CO2 efflux rates: is it caused by low stem turgor pressure? Ann Bot (Lond) 99:477–485. doi:10.1093/aob/mcl268
Saveyn A, Steppe K, Lemeur R (2008a) Report on non-temperature related variations in CO2 efflux rates from young tree stems in the dormant season. Trees (Berl) 22:165–174. doi:10.1007/s00468-006-0099-6
Saveyn A, Steppe K, McGuire MA, Lemeur R, Teskey RO (2008b) Stem respiration and carbon dioxide efflux of young Populus deltoides trees in relation to temperature and xylem carbon dioxide concentration. Oecologia 154:637–649. doi:10.1007/s00442-007-0868-y
Schrumpf M, Guggenberger G, Schubert C, Valarezo C, Zech W (2001) Tropical montane rain forest soils—development and nutrient status along an altitudinal gradient in the south Ecuadorian Andes. Erde 132:43–59
Sorz J, Hietz P (2006) Gas diffusion through wood: implications for oxygen supply. Trees (Berl) 20:34–41. doi:10.1007/s00468-005-0010-x
Sprugel DG, Benecke U (1991) Measuring woody-tissue respiration and photosynthesis. In: Lassoie JP, Hinckley T (eds) Techniques and approaches in forest tree ecophysiology. CRC-Press Inc, Boston, pp 329–355
Steppe K, Saveyn A, McGuire MA, Lemeur R, Teskey RO (2007) Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux rates of Populus deltoides stems. Funct Plant Biol 34:785–792. doi:10.1071/FP07077
Stockfors J, Linder S (1998) Effect of nitrogen on the seasonal course of growth and maintenance respiration in stems of Norway spruce trees. Tree Physiol 18:155–166
Strobel J (2004) Die Atmung der verholzten Organe von Altbuchen (Fagus sylvatica L.) in einem Kalk- und einem Sauerhumusbuchenwald. Unpublished PhD thesis, University of Göttingen, Germany
Teskey RO, McGuire MA (2002) Carbon dioxide transport in xylem causes errors in estimation of rates of respiration in stems and branches of trees. Plant Cell Environ 25:1571–1577. doi:10.1046/j.1365-3040.2002.00961.x
Teskey RO, McGuire MA (2007) Measurement of stem respiration of sycamore (Plantanus occidentalis L.) trees involves internal and external fluxes of CO2 and possible transport of CO2 from roots. Plant Cell Environ 30:570–579. doi:10.1111/j.1365-3040.2007.01649.x
Teskey RO, Saveyn A, Steppe K, McGuire MA (2008) Origin, fate and significance of CO2 in tree stems. New Phytol 177:17–32
Tjoelker MG, Oleksyn J, Reich PB (1999) Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Glob Change Biol 49:679–691. doi:10.1046/j.1365-2486.1999.00257.x
Tjoelker MG, Oleksyn J, Reich PB (2001) Modelling respiration of vegetation: evidence for a general temperature-dependent Q 10. Glob Change Biol 7:223–230. doi:10.1046/j.1365-2486.2001.00397.x
Zach A, Horna V, Leuschner C (2008) Elevational changes in woody tissue CO2 efflux rates in a tropical mountain rainforest in southern Ecuador. Tree Physiol 28:67–74
Acknowledgments
This study was funded by the DFG (German Science Foundation) through a grant in the Research Unit 402 (Functionality in a Tropical Mountain Rainforest, subproject B6). We gratefully acknowledge the Ministerio del Ambiente Loja-Zamora for the research permit, and the Fundación Científica San Francisco (Nature and Culture International) for the ongoing support at Estación Científica San Francisco. We thank Florian Werner (University of Goettingen) for invaluable contributions on early versions of the manuscript. We are also grateful to Stefan Engelhardt (University of Bayreuth), Rüttger Rollenbeck (University of Marburg), Michael Richter and Thorsten Peters (University of Erlangen) as well as to Gerald Moser (University of Goettingen) for contributing data on soil water, climate and general stand characteristics, respectively.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Pfanz.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Zach, A., Horna, V. & Leuschner, C. Diverging temperature response of tree stem CO2 release under dry and wet season conditions in a tropical montane moist forest. Trees 24, 285–296 (2010). https://doi.org/10.1007/s00468-009-0398-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-009-0398-9