Abstract
Purpose
Remifentanil has been available in Japan for 3 years. The use of this new opioid is considered a useful adjuvant to general anesthesia. Knowing the exact cost-effectiveness of remifentanil should lead to improved anesthetic outcomes with a reasonable cost.
Methods
This single-blinded, prospective, randomized study compared the cost of remifentanil-based general anesthesia combined with isoflurane, sevoflurane, or propofol with fentanyl-based conventional techniques in 210 women who underwent breast surgeries.
Results
Remifentanil-based general anesthesia was no more expensive than fentanyl-based conventional anesthesia. Postoperative nausea and vomiting was significantly less frequent after remifentanil-based than fentanyl-based anesthesia.
Conclusion
This study shows that remifentanil-based general anesthesia is no more expensive than conventional fentanyl-based anesthesia under the Japanese health care system because of the small difference in price between remifentanil and fentanyl.
Similar content being viewed by others
Introduction
Under its unique universal health insurance system, Japan has maintained the cost of national health care expenditure at 8.1% of the gross domestic product (GDP) while offering efficient health care services to its citizens [1]. Under this system, large health care facilities tend to use the diagnosis procedure combination (DPC) system. On the other hand, smaller facilities tend to use the fee-for-services (FFS) system, and the cost of anesthesia is calculated using the FFS system with authorized prices. Because of this system, Japanese anesthesiologists usually have little interest in, and have rarely studied, medical economics of anesthesia. However, the Japanese government, which pays 81.3% of the health care costs based on the Japanese public insurance system, recently initiated programs to cut huge medical costs and revenue deficit. Therefore, it is possible that the DPC system will be employed to calculate the cost of anesthesia in the near future. In such situations, it seems important for anesthesiologists to recognize the cost-effectiveness of various anesthesia methods.
Remifentanil is an extremely useful anesthetic adjunct, as its continuous infusion provides stable anesthesia and patient hemodynamics as well as quick metabolism [2]. Since remifentanil was introduced in Japan in 2007, general anesthesia using remifentanil has become the mainstream anesthetic technique. In contrast, the market share of remifentanil remains very small in the European Union (EU) and the United States despite the fact that it was introduced more than 10 years ago in the USA, as remifentanil-based anesthesia is much more expensive than conventional fentanyl-based conventional anesthesia [3]. Actually, many studies in Western countries have concluded that anesthesia using remifentanil is expensive, although the quality of the anesthesia is high [4].
Official prices of anesthetic-associated drugs are shown in Table 1. The cost-effectiveness of remifentanil in Japan might be different from that in Western countries. In Japan, one 2-mg vial of remifentanil is just seven times more expensive than a 2-mL ampoule of fentanyl, whereas one 2-mg vial of remifentanil is >130 times more expensive than a 2 mL ampoule of fentanyl in the USA (Tables 1, 2). Although remifentanil has been available in Japan for more than 3 years, knowing its exact cost-effectiveness may lead to improved anesthetic outcomes with a reasonable cost. An economic analysis on remifentanil in comparison with conventional techniques has not yet been reported in Japan. Therefore, the purpose of this study was to compare the costs and outcomes of newer remifentanil-based anesthesia with those of conventional fentanyl-based anesthesia in the same patient population to evaluate exact cost-effectiveness of remifentanil in Japan.
Materials and methods
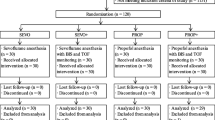
This single-blinded, prospective, randomized study was approved by the institutional review board at the Cancer Institute Hospital, Tokyo, Japan. A total of 210 American Society of Anesthesiologists (ASA) physical status I and II women 20 years or older and scheduled for breast surgery participated in this study. Written informed consent was obtained from each patient. We chosen breast surgery patients because >1,000 breast surgeries were conducted during the study period. The majority of these patients were healthy except for the conditions that required breast surgery, and the duration of surgery and postoperative pain were both uniformly moderate. Coronary or valvular heart disease, renal insufficiency (creatinine >1.2 mg/dL), liver dysfunction [aspartate aminotransferase (AST) and alanine aminotransferase (ALT) >100 U/L], abuse of alcohol or drugs, psychiatric diseases, and a body mass index (BMI) >35 kg/m2 were defined as exclusion criteria. Two nationally certified anesthesiologists participated in this study. No patients were given premedication before the anesthesia. Patients were randomly assigned to one of seven groups. The number of patients in each group was 30. All patients were monitored by electrocardiogram, pulse oximetry (SpO2), heart rate derived from SpO2 monitoring, noninvasive blood pressure, continuous capnography (EtCO2), anesthetic gas monitoring (model BSM-5132, Nihon Kohden Corporation, Japan), and the Bispectral (BIS) index (model A-2000, Aspect Medical System, USA). All measurements were recorded every 5 min using a medical monitoring system. Both the inspired concentration of inhalational anesthetic agents and the continuous infusion of propofol with the target-controlled infusion (TCI) pump (model TE371, TERUMO Corporation, Japan) were used to maintain the BIS index at 40–60.
During the anesthetic induction, 100% oxygen flow was maintained at 6 L/min. Immediately after orotracheal intubation, the constant fresh gas flow of 3 L/min was used during the maintenance of anesthesia using sevoflurane, because the US Food and Drug Administration (FDA) reported that the total fresh gas flow should not be below 2 L/min during administration of sevoflurane [5] to minimize the risk of nephrotoxic compound A generation, which could harm the kidneys [6]. In isoflurane and propofol anesthesia, the constant fresh gas flow was 1 L/min. All ventilation and vaporization of volatile gases were performed using an Aestiva 5 (Datex-Ohmeda, USA). All patients maintained SpO2 at >98% and EtCO2 at 35–40 mmHg. Fentanyl TCI was simulated using the Palmacokinetics Version 0.98, created by Dr. Osamu Uchida (http://homepage1.nifty.com/o-uchida/palmacokinetics/), installed into the Palm Z22, a personal digital assistant (Palm Inc., USA). Bicarbonated Ringer’s and colloid solutions were infused in all patients.
In group N2O/sevoflurane/fentanyl (NSF), a conventional technique group, anesthesia was induced using 100 μg fentanyl, vecuronium bromide at 0.1–0.15 mg/kg, and propofol at 2 mg/kg and was then maintained using nitric oxide (N2O) (66.7%), sevoflurane (1.0–2.0%), and fentanyl TCI intravenously at 1.0–1.5 ng/mL. Vecuronium bromide was added only in the case of patient movement. Fentanyl TCI was titrated at not more than 1.0 ng/mL at extubation after the operation. In group sevoflurane/fentanyl (SF), another conventional group, the same induction procedure as in group NSF was used, except that N2O was not included for maintenance. In group sevoflurane/remifentanil (SR), a new technique group, anesthesia was induced by continuous infusion of remifentanil at 0.5 μg/kg/min, vecuronium bromide at 0.1–0.15 mg/kg, and propofol at 1 mg/kg and was maintained using sevoflurane (1.0–2.0%) and continuous infusion of remifentanil at 0.2 μg/kg/min. Fentanyl was administrated intravenously for postoperative analgesia after the specimen was completely removed while the continuous infusion of remifentanil at 0.2 μg/kg/min continued, which did not exceed 1.0 ng/mL of TCI at the end of each operation. In group N2O/isoflurane/fentanyl (NIF), a conventional group, the protocol used for group NSF was also employed, but 60% N2O and isoflurane (0.5–1.0%) were administered for maintenance. In group isoflurane/remifentanil (IR), a new technique group, the same procedure used for group SR was employed, but isoflurane (0.5–1.0%) was administered for maintenance of anesthesia. In group propofol/fentanyl (PF), a conventional total intravenous anesthesia (TIVA) group, anesthesia was induced using fentanyl at 100 μg, vecuronium bromide at 0.1–0.15 mg/kg, and propofol TCI at 4.0 μg/mL, and was maintained using propofol at 2.0–4.0 μg/mL and fentanyl TCI intravenously at 1.0–1.5 ng/mL. Fentanyl TCI was titrated at not more than 1.0 ng/mL at extubation after the operation. In group propofol/remifentanil (PR), a new TIVA-technique group, anesthesia was induced by continuous infusion of remifentanil at 0.5 μg/kg/min, vecuronium bromide at 0.1–0.15 mg/kg, and propofol TCI at 3.0 μg/mL, and was maintained by propofol TCI (2.0–4.0 μg/mL) and continuous infusion of remifentanil at 0.2 μg/kg/min. The same procedure for fentanyl administration as postoperative analgesia in group SR and IR was also employed.
In all cases, before the surgeons removed the specimen completely, we maintained patients’ systolic blood pressure at 80–100 mmHg, controlling volatile gas anesthetics or propofol TCI to reduce blood loss. After surgery, we targeted patients’ ordinary systolic pressure in the ward using ephedrine or phenylephrine hydrochloride injection to help surgeons investigate for bleed or oozing in the operated region. Flurbiprofen axetil 50 mg ampoule, a nonsteroidal anti-inflammatory drug (NSAID), was administered intravenously for patients without NSAID hypersensitivity during the operation. Additional injections of a muscle relaxant were administered in a few cases.
At the completion of the surgical procedures, all anesthetic and analgesic agents were discontinued. Next, 100% oxygen was administered at 6 L/min until the patient was extubated, when the anesthesiologist confirmed that she opened their eyes and gripped their hands on command. After extubation, all patients were observed for at least 10 min in the operating room. After the anesthesiologist confirmed recovery, the patient was transferred to an ordinary ward. Immediately after each case, the attending anesthesiologist recorded the patient’s intraoperative course and complications, if any, using a brief survey. Postoperative nausea and vomiting (PONV) was treated in the ward by the attending surgeons. The attending anesthesiologist surveyed the patient’s postoperative condition through clinical records and checked for PONV. We also surveyed the patients regarding satisfaction with their anesthesia on a 3-point Likert scale the day after the operation. The Likert scale ranged from “satisfied” to “dissatisfied,” with 3 being satisfied (better than expected), 2 being neutral, and 1 being dissatisfied (worse than expected). Except for the volatile agents, all drug costs were taken from the Official Price List of Pharmaceuticals under the social insurance system in Japan. Our cost analysis did not include the cost of running equipment, supplies, consumables, and the physician’s fee. The consumption of volatile anesthetics was calculated using the following official Japanese formula:
where F means fresh gas flow (L/min) and C is the monitored concentration (%). All variables and cost data were compared in all groups using Welch’s t test, analysis of variance (ANOVA), and the Kruskal–Wallis test. Data are presented as mean ± standard deviations (SD). P < 0.05 were considered statistically significant.
Results
There were no significant differences in age, height, weight, BMI, ASA physical status (Table 3), vital signs before anesthesia, intraoperative fluid volume per minute, blood loss, urine volume, surgical duration, and anesthetic period between the groups. The adverse events during anesthesia were also similar.
-
1.
Remifentanil versus fentanyl as an adjuvant to sevoflurane
Although the total anesthetic cost was similar in groups NSF, SF, and SR, the calculated consumption of sevoflurane was greater in group SF than groups NSF and SR by more than 20 mL per operation. PONV was significantly less, and the level of patient satisfaction was significantly more in group SR than groups NSF and SF (Table 4).
Table 4 Remifentanil versus fentanyl as an adjuvant to sevoflurane -
2.
Remifentanil versus fentanyl as an adjuvant to isoflurane
The total cost was lower in group NIF than in group IR, and the consumption of isoflurane was larger in group NIF than in group IR. However, PONV occurred significantly less frequently in group IR than in group NIF (Table 5).
Table 5 Remifentanil versus fentanyl as an adjuvant to isoflurane -
3.
Remifentanil versus fentanyl as an adjuvant to propofol
Although there were no significant differences in total cost, the volume of propofol consumed was greater in group PF than in group PR by approximately 500 mg. There were no significant differences in the incidence of PONV or patient satisfaction in relation to anesthesia (Table 6).
Table 6 Remifentanil versus fentanyl as an adjuvant to propofol -
4.
Comparison of remifentanil-based anesthesia groups
The total cost for group IR was significantly less than the other groups. Postoperative PONV and patient satisfaction showed no significant differences between the remifentanil-based groups (Tables 4, 5, 6).
-
5.
Comparison of fentanyl-based anesthesia groups
The total cost of group NIF was significantly less than the other groups. In group PF, PONV appeared significantly less often in comparison with the other fentanyl-based groups (Tables 4, 5, 6).
-
6.
Comparison of all groups
The total cost was lowest in group NIF and highest in group PR (Tables 4, 5, 6).
Discussion
Remifentanil-based anesthesia has been reported to be more expensive than fentanyl-based conventional anesthesia in Western countries. However, the results of this study are different from traditional clinical economic findings reported in Western countries. In our study, when sevoflurane was used, there were no differences in the total and per-minute costs of anesthetics between patients who received remifentanil and those who received fentanyl. These results are different from those reported by Abenstein et al. [7], and the difference may be related to the public health insurance system in Japan. When we looked at the quality of anesthesia, remifentanil had a significantly lower frequency of PONV and a significantly higher level of patient satisfaction. Therefore, remifentanil increases cost-effectiveness when used in conjunction with sevoflurane.
In the group of patients receiving isoflurane, remifentanil use was associated with a significantly higher cost, but the quality of anesthesia was again very high. In the group of patients receiving TIVA, there was no significant difference in cost and quality compared with more traditional methods. Although the cost-effectiveness of remifentanil is not high, it may improve for longer surgeries, as less propofol is used per body weight. When remifentanil use in the three different groups was considered, the total cost of the combination of isoflurane/remifentanil was cheaper than the other two methods by approximately ¥2,500 (US $27). We expected this result, as isoflurane is more suitable than sevoflurane for low-flow anesthesia, and the minimum alveolar concentration (MAC) is low. TIVA has been reported to be expensive [8], but there was no detectable difference in our study. Sevoflurane use tends to be more expensive, possibly because a high-flow method is recommended. If we can completely ignore the production of nephrotoxic compound A [9] and use the low-flow method (lower than 1 L/min), even when sevoflurane is used, the cost of anesthesia will become lower than that of TIVA [10]. There was no difference in anesthesia quality among the three remifentanil methods. PONV was observed in only two cases out of 90, and the quick metabolism of remifentanil is considered to be responsible for lowering the frequency of such side effects. When fentanyl use in the four groups is considered, isoflurane use was cheaper than the other methods by approximately ¥3,600. The total cost of sevoflurane anesthesia depends on whether N2O is used, as N2O is expensive in Japan. N2O use should be minimized, as it is not cost-effective and contributes to destruction of the ozone layer [11]. The use of volatile anesthetic gas in combination with fentanyl significantly increases the incidence of PONV [12, 13]. Propofol has been reported to have an antiemetic effect, and our results support this conclusion [14]. It is difficult to define an anesthetic method that would increase the cost-effectiveness of fentanyl use based on our study results.
When isoflurane use in two groups is considered, remifentanil use was more expensive than fentanyl. It was due to the small amount of isoflurane and N2O used in low-flow anesthesia at 1 L/min. N2O use proved expensive in sevoflurane use; however, the low-flow technique might be a major reason to decrease the total anesthetic cost in volatile gas use. As remifentanil generally has a low incidence of PONV, the cost of postoperative care should be reduced. If Japanese hospitals use the DPC system more frequently, cost-effectiveness will further increase.
In this study, we investigated anesthetic techniques used in breast surgeries and concluded that the use of remifentanil is no more expensive than the traditional anesthetics based on total costs. As the high quality of this anesthesia lowers the cost of postoperative care, remifentanil is a highly cost-effective anesthetic in the Japanese health care system.
References
Organization of Economic Co-operation and Development. OECD health data 2009. Health expenditure. http://stats.oecd.org/Index.aspx?DatasetCode=HEALTH
Servin FS, Billard V. Remifentanil and other opioids. Handb Exp Pharmacol. 2008;182:283–311.
Beers RA, Calimlim JR, Uddoh E, Esposito BF, Camporesi EM. A comparison of the cost-effectiveness of remifentanil versus fentanyl as an adjuvant to general anesthesia for outpatient gynecologic surgery. Anesth Analg. 2000;91:1420–5.
Loop T, Priebe HJ. Prospective, randomized cost analysis of anesthesia with remifentanil combined with propofol, desflurane or sevoflurane for otorhinolaryngeal surgery. Acta Anaesthesiol Scand. 2002;46:1251–60.
United States Food and Drug Administration. Ultane/Sevoflurane. NDA 20-478/S-006. http://129.128.185.122/drugbank2/drugs/DB01236/fda_labels/194. Accessed 5 Aug 2010.
Kharasch ED, Schroeder JL, Bammler T, Beyer R, Srinouanprachanh S. Gene expression profiling of nephrotoxicity from the sevoflurane degeneration product fluoromethyl-2,2-difluoro-1-(trifluoromethyl)vinyl ether (“compound A”) in rats. Toxicol Sci. 2006;90:419–31.
Abenstein JP, Long KH, McGlinch BP, Dietz NM. Is physician anesthesia cost-effective? Anesth Analg. 2004;98:750–7.
Boldt J, Jaun N, Kumle B, Heck M, Mund K. Economic considerations of the use of new anesthetics: a comparison of propofol, sevoflurane, desflurane, and isoflurane. Anesth Analg. 1998;86:504–9.
Croinin DF, Shorten GD. Anesthesia and renal disease. Curr Opin Anaesthesiol. 2002;15:359–63.
Lockwood GG, White DC. Measuring the cost of inhaled anaesthetics. Br J Anaesth. 2001;87:559–63.
Ravishakara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–5.
Fernandez-Guisasola J, Gomez-Amau JI, Cabrera Y, Del Valle SG. Association between nitrous oxide and the incidence of postoperative nausea and vomiting in adults: a systematic review and meta-analysis. Anaesthesia. 2010. doi:10.1111/j.1365-2044.2010.06249.x.
Gan TJ. Risk factors for postoperative nausea and vomiting. Anesth Analg. 2006;102:1884–98.
Unal Y, Ozsoylar O, Arslan M, Sarigüney D, Akçabay M. Comparison of the efficacy of propofol and metoclopramide in preventing postoperative nausea and vomiting after middle ear surgery. Saudi Med J. 2009;30:778–82.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nakada, T., Ikeda, D., Yokota, M. et al. Analysis of the cost-effectiveness of remifentanil-based general anesthesia: a survey of clinical economics under the Japanese health care system. J Anesth 24, 832–837 (2010). https://doi.org/10.1007/s00540-010-1006-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-1006-2