Abstract
Insomnia symptoms are frequent during peripartum and are considered risk factors for peripartum psychopathology. Assessing and treating insomnia and related conditions of sleep loss during peripartum should be a priority in the clinical practice. The aim of this paper was to conduct a systematic review on insomnia evaluation and treatment during peripartum which may be useful for clinicians. The literature review was carried out between January 2000 and May 2021 on the evaluation and treatment of insomnia during the peripartum period. The PubMed, PsycINFO, and Embase electronic databases were searched for literature published according to the PRISMA guidance with several combinations of search terms “insomnia” and “perinatal period” or “pregnancy” or “post partum” or “lactation” or “breastfeeding” and “evaluation” and “treatment.” Based on this search, 136 articles about insomnia evaluation and 335 articles on insomnia treatment were found and we conducted at the end a narrative review. According to the inclusion/exclusion criteria, 41 articles were selected for the evaluation part and 22 on the treatment part, including the most recent meta-analyses and systematic reviews. Evaluation of insomnia during peripartum, as for insomnia patients, may be conducted at least throughout a clinical interview, but specific rating scales are available and may be useful for assessment. Cognitive behavioral therapy for insomnia (CBT-I), as for insomnia patients, should be the preferred treatment choice during peripartum, and it may be useful to also improve mood, anxiety symptoms, and fatigue. Pharmacological treatment may be considered when women who present with severe forms of insomnia symptoms do not respond to nonpharmacologic therapy.
Similar content being viewed by others
Introduction
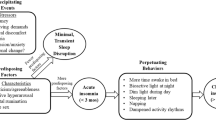
Sleep is an important regulatory psychophysiological behavior in life, influencing mood, emotion, and impulse behaviors, which are key mediators of stress adjustments so commonly needed in the perinatal period (Baglioni et al. 2020). Consistently, sleep problems are recognized as a major risk factor for mental and physical health problems (Palagini et al. 2013; Hertenstein et al. 2019) and sleep is commonly impaired during peripartum (Palagini et al. 2014; Mindell et al. 2015; Pengo et al. 2018; Garbazza et al. 2020). Women’s sleep during pregnancy and post-partum is altered by anatomical, endocrinology, physiological, psychological, behavioral, socioeconomic, and cultural factors (Pengo et al. 2018). With the physical and hormonal adaptations in pregnancy, changes in sleep are reported by 66 to 97% of women (Balserak and Lee 2017; Kay-Stacey et al. 2017) with 75–98%, of during the third trimester of pregnancy (Palagini et al. 2014; Balserak and Lee 2017; Kay-Stacey and Attarian 2017; Baglioni et al. 2020a, 2020b, 2020c; Swanson et al. 2020). Most common problems during all three trimesters include short sleep duration, poor sleep quality, conditions of sleep loss, and insomnia (Palagini et al. 2014; Mindell et al. 2015; Pengo et al. 2018; Garbazza et al. 2020; Baglioni et al. 2020a, 2020b, 2020c; Swanson et al. 2020). In particular, insomnia may affect more that 50% of the pregnant women reaching until the 80% of women during the third trimester (Swanson et al. 2020; Sedov et al. 2021). Vulnerability to insomnia is greatly heightened during the perinatal period with racial disparity to endorse the insomnia symptoms (Swanson et al. 2020). According to the “3-P” model of insomnia with predisposing, precipitating, and perpetuating factors relevant to the development and maintenance of insomnia (Riemann et al. 2015), hormonal and physical factors may predispose pregnant women to develop insomnia in response to pregnancy related emotional distress (Palagini et al. 2014; Balserak and Lee 2017; Kay-Stacey and Attarian 2017; Pengo et al. 2018; Baglioni et al. 2020a, 2020b, 2020c; Swanson et al. 2020). Then, maladaptive sleep behaviors together with other sleep disorders such as sleep disorders breathing (SDB) and restless leg syndrome (RLS) which are frequently experienced during the last trimester of pregnancy may perpetuate insomnia in pregnancy (Kalmbach et al. 2019; Swanson et al. 2020). These factors may fuel the cycle of hyperarousal in insomnia with hyperactivation of stress and inflammatory systems (Riemann et al. 2010, 2015) leading to stress system allostatic “overload” which may account for adverse pregnancy outcomes including peripartum psychopatology (Palagini et al. 2014; Swanson et al. 2020; Swanson et al. 2020; Sharma et al. 2021). Cumulative evidence points out that disrupted sleep in pregnancy including insomnia may be linked to negative gestational and birth outcomes, emergency cesarean section, gestational diabetes (Okun et al. 2011; Anothaisintawee et al. 2016; Paine et al. 2020), and most importantly are risk factors for peripartum psychopathology. Insomnia and disrupted sleep have considered a risk factor for unipolar and bipolar depression during pregnancy and postpartum (Sharma and Mazmanian 2003; Tomfohr et al. 2015; Palagini et al. 2014; Emamian et al. 2019; Baglioni et al. 2020a, 2020b, 2020c; Kalmbach et al. 2020a, 2020b, 2020c; Swanson et al. 2020; Sedov et al. 2021; Kalmbach et al. 2021a, 2021b, Sharma et al. 2021). Insomnia symptoms in early pregnancy may predict depressive symptoms in late pregnancy and sleep disturbances in late pregnancy have shown to independently predicting symptoms of post-partum depression (Tomfohr et al. 2015; Palagini et al. 2014; Emamian et al. 2019). In addition, insomnia symptoms during pregnancy may mediate the relation between post-partum blues and increased risk of postpartum depression (Ross et al. 2005). Most importantly, insomnia symptoms during peripartum have linked to an increased suicidal risk (Palagini et al. 2019; Kalmbach et al. 2020a, 2020b, 2020c). Sharma and Mazmanian (2003) have discussed that sleep loss/disruption may be the final common pathway in the development of postpartum psychotic episodes.
Maternal sleep patterns in pregnancy may also affect infant sleep patterns, such that disrupted maternal sleep in pregnancy is associated with worse infant sleep, which can in turn further disrupt maternal postpartum sleep (Meltzer and Montgomery-Downs 2011; Mindell et al. 2017). Sleep in the perinatal period has been considered a family issue with potential long-term consequences modifying child’s vulnerability to mental health during adult life (Mindell et al. 2017; Baglioni et al. 2020a, 2020b, 2020c).
In this framework, assessing and treating insomnia and related conditions of sleep loss during peripartum should be a priority in the clinical practice. It might reduce the risk for postpartum psychopathology (Sharma et al. 2021). Alternatively, the regulation of sleep–wake patterns could offer relief to women in whom symptoms of these disorders have already developed. In this context, the main aim of this paper was to conduct a systematic review on insomnia evaluation and treatment during peripartum, which may be useful for clinicians in the clinical practice. The European Insomnia Network task force on “Sleep and Women” promoted the work and it represents a joint position paper with the Italian Marcè Society for Perinatal Mental Health and with internationally recognized experts in peripartum psychopathology. The aim of the project was to optimize evaluation and treatment of insomnia and related conditions of disrupted sleep during peripartum in the clinical practice.
Method
The literature review was carried out up from January 2000 to May 2021 on the evaluation and treatment of insomnia during the peripartum period including pregnancy, postpartum and lactation.
Information sources The PubMed, PsycINFO, and Embase electronic databases were searched for literature published according to the PRISMA (preferred reporting items for systematic reviews and meta-analysis) method (Moher et al. 2009). Searches were performed by LP and CB. Results were synthesized by LP. Search strategy was conducted using keywords relating to insomnia and perinatal period. The literature search was conducted on electronic databases [Medline (Ovid), Web of Science (Core), Embase (Ovid), PsychInfo (Ebsco) and PsychArticles (Ebsco)] between January 2000 and May 2021. The search strategy was developed using keywords and medical subject heading terms (MeSH) to encompass insomnia assment and evaluation during peripartum.
Search strategy
Several combinations of search terms were used such as “insomnia” and “perinatal period” or “pregnancy” or “post partum” or “lactation” or “breastfeeding” and “evaluation” and “treatment” were included.
Selection process
Inclusion criteria were studies. (1) Only studies and reviews that included participants during pregnancy and postpartum periods were eligible for inclusion. (2) Interested insomnia in pregnant women or women during the postpartum period. (3) Full-text studies published in English in peer-reviewed journals were eligible for inclusion in the review. Systematic reviews and meta-analyses were included. Papers were excluded if they concerned other sleep disorders such for example sleep disorder breathing or restless leg syndrome, or studies evaluating sleep quality, studies including complementary and alternative medicine for insomnia which are not recommended for insomnia treatment (Riemann et al. 2017).
Outcome measures
The main outcome of interest of this review was how to evaluate and treat insomnnia symptoms during pregnancy and post partum.
Study design
All studies that explored an association between insomnia and pregnancy or postpartum were included in the review.
Assessment of risk of bias
Quality of studies, reviews, and methanalyses was checked; a decision was taken to only include studies that utilised validated measures of insomnia while other forms of assessment were removed. We expected eterogenities to represent a risk of bias. At the end, due to eterogenity of the studies, we produced a narrative review, accompanied by tabulated details of the included studies.
Results
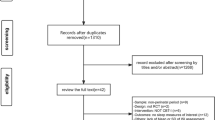
Based on the systematic search, 136 articles about insomnia evaluation and 335 articles about insomnia treatment were found. According to the inclusion/exclusion criteria, 41 articles were selected for the evaluation of insomnia and 22 on the treatment part included most recent meta-analyses and systematic reviews (Fig. 1).
Evaluation of insomnia during pregnancy and postpartum
According to international guidelines, insomnia evaluation needs a patient history and examination addressing sleep and waking functions as well as common medical, psychiatric, and medication/substance-related comorbidities (Sateia et al. 2017; Riemann et al. 2017; Palagini et al. 2020). International guidelines suggest evaluating insomnia symptoms using the Consensus Sleep Diary for at least 1/2 weeks to assess the insomnia day-to-day variability (Carney et al. 2012; Sateia et al. 2017; Riemann et al. 2017; Palagini et al. 2020). In addition, the administration of questionnaires and survey instruments has been suggested to assesses outcomes and to guiding treatment including the Insomnia Severity Index (ISI) (Morin 1993) and the Epworth Sleepiness Scale (ESS) (Johns 1991) (Riemann et al. 2017; Palagini et al. 2020). These questionnaires have been extensively used in the evaluation of insomnia during the peripartum period across different countries, for an overview, see Table 1. In Table 1, we can observe heterogeneity among studies but the majority of studies used ISI to evaluate insomnia during the perinatal period (Table 1), sleep diary has been also used frequently to assess the insomnia day-to-day variability during pregnancy (Table 1); ESS has been used in some studies detecting daytime insomnia symptom in peripartum (Table 1). Other questionnaires which have been used to evaluated insomnia during peripartum were the Bergen Insomnia Scale (Pallesen et al. 2008) but it was used in studies from Norway only, the Insomnia Symptom Questionnaire (ISQ) which is an insomnia questionnaire validated among pregnant women (Okun et al. 2015) but it has been used in two studies only (Sedov et al. 2021). Additional evaluations during pregnancy have included the measure of stress related sleep reactivity with the Ford Insomnia Response to Stress Test (FIRST) (Drake et al. 2004) to measure the vulnerability to insomnia and psychopathology during pregnancy (Gelaye et al. 2016; Palagini et al. 2019; Gelaye et al. 2016; Sanchez et al. 2020). Particularly for the evaluation of perpetuating negative behaviors and cognitive processes, the Dysfunctional Beliefs and Attitudes About Sleep Scale (DBAS) (Morin 1993) that is suggested for insomnia has been used in one study during pregnancy (Wang et al. 2020) and the pre-sleep arousal which may perpetuate insomnia with Pre-sleep Arousal Scale (Nicassio et al. 1985) in 4 studies (Table 1). Sleep quality has been extensively measured with the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al. 1989) that should be useful for evaluation of sleep duration and other sleep disorders in pregnancy. In particular, during peripartum, it is of importance to assess SDB and RLS, which are frequently experienced during the last trimester of pregnancy and may be related to insomnia symptoms (for an overview see Sedov et al. 2018); indeed, the majority of the studies did not assessed these sleep disorders. The Nordic Basic Sleep Questionnaire (Partinen and Gislason 1995) was also used to evaluate insomnia and other sleep disorders during peripartum (Table 1) but in studies conducted in Finland only.
Both polysomnographic and actigraphic registration are not recommended for the routine evaluation of insomnia. They are suggested if other sleep disorders are reasonably suspected to be related to insomnia. Particularly, actigraphic has been used in few studies for insomnia evaluation during pregnancy (Table 1), while no studies used polysomnographic registration in insomnia during peripartum.
Management of insomnia during peripartum
Timely assessment and appropriate management are essential to prevent potential adverse pregnancy outcomes and re-occurrence of chronic insomnia (Sharma et al. 2021). It is of importance to know that many pregnant women do not seek treatment for insomnia, because they think either it will naturally resolve after birth or wish to avoid medication owing to concerns about adverse effects on the fetus (Kay-Stacey and Attarian 2017). If therefore, it seems of utmost importance to clinically assess and manage sleep disruption from the beginning of pregnancy. The National Institute for Health and Clinical Excellence (NICE) guideline on antenatal and postnatal mental health 2018 recommends that wherever possible, psychological therapies (supportive psychotherapy, cognitive behavioral therapy and interpersonal therapy) should be the first-line treatment for mild to moderate conditions. The threshold for using psychotropic medication should be relatively high and it should be prescribed only if a psychological approach alone does not alleviate symptoms (NICE 2018).
For chronic insomnia, the cognitive behavioral therapy for insomnia (CBT-I) is the internationally considered first-line treatment (Riemann et al. 2017; Palagini et al. 2020; Bacaro et al. 2020; Baglioni et al. 2020a, 2020b, 2020c; Baglioni and Palagini 2021).
Cognitive behavioral therapy for insomnia (CBT-I) during pregnancy and postpartum
Cognitive behavioral therapy for insomnia usually consists of behavioral strategies including psycho-education/sleep hygiene, relaxation training, stimulus control therapy, sleep restriction therapy, and cognitive strategies such as sleep/related cognitive restructuring (Baglioni et al. 2020a, 2020b). In the context of CBT-I, psycho education typically includes the so-called sleep hygiene rules about health practices and environmental factors (e.g., light, noise, temperature) that may promote or disrupt sleep. Relaxation therapy is aimed at reducing somatic tension or intrusive thoughts at bedtime. Behavioral strategies include sleep restriction and stimulus control therapies; sleep restriction is a method designed to curtail the time in bed to the actual amount of sleep being achieved and stimulus control therapy is a set of behavioral instructions designed to re-associate the bed/bedroom with sleep and to re-establish a consistent sleep–wake schedule. In summary, CBT-I may be effective, because it increases sleep drive, extinguishes conditioned arousal, and focuses on altering maladaptive behaviors and cognitions that perpetuate poor sleep (Baglioni et al. 2020b, 2020c). A recent systematic review pointed out a severe lack of knowledge on effective clinical interventions for insomnia during pregnancy (Bacaro et al. 2020). The review selected 16 studies including in total 1252 expecting mothers. Four studies evaluated cognitive behavioral interventions for insomnia, one study pharmacotherapy, one study acupuncture, three studies mindfulness or yoga, five studies relaxation techniques, and two studies herbal medication. Of those, only six were randomized controlled trials. Preliminary support was evidenced for cognitive behavioral interventions for insomnia (Table 2), which was also found to be the preferred therapy for pregnant women compared to pharmacological therapy (Sedov et al. 2017). Indeed, some promising data come from studies using mindfulness (Kalmbach et al. 2019). CBT-I should be the preferred choice during peripartum because it is the first-line treatment for insomnia and, particularly, according to NICE guideline during peripartum wherever possible, psychological/non pharmacological therapies including, cognitive behavioral therapy, should be first-line treatment for mild to moderate conditions.
In 2017, Tomfohr-Madsen conducted a study investigated the effectiveness of group cognitive-behavioral therapy for insomnia (CBT-I) delivered in pregnancy. Thirteen pregnant women with insomnia participated in five weekly CBT-I group sessions and showed an improvement in sleep latency, sleep efficiency, and increased subjective total sleep time but also in symptoms of depression, pregnancy-specific anxiety, and fatigue. Four randomized controlled studies evaluated efficacy of psychological interventions for sleep difficulties during pregnancy. Tested experimental interventions included 4 session-therapy including sleep hygiene education (SHE) and instructions for stimulus control (Rezaei et al. 2014); 5-session CBT-I including SHE, stimulus control, strategies for reducing cognitive and somatic arousal, and modified sleep restriction therapy (SRT) (Manber et al. 2019), and 6-session digital CBT-I (using Sleepio) including standard protocol with adapted SRT (Felder et al. 2020; Kalmbach et al. 2020a, 2020b, 2020c). In total, 278 expectant mothers received experimental interventions compared to 267 pregnant women receiving control interventions. All together, these studies point out that CBT-I improves maternal sleep and related mood symptoms and SRT should be adapted to minimize related stress and fatigue, which may be not indicated during pregnancy. Specific adaptation of the standard cognitive-behavioral therapy for insomnia protocol for pregnant women has been proposed to improving sleep hygiene, with sleep psychoeducation focusing on specific aspects of pregnancy and post-partum. Strategies targeting emotional aspects may be stressed and get a more central role compared to standard CBT-I protocol. Family issues may be taken into consideration together with balance between working and family lives (Baglioni 2020). Swanson et al. (2020) pointed out that when prescribing sleep schedules during pregnancy is better to never reduced the sleep window to less than 6 h, and provide flexibility in bed/wake-times with bed- and wake-time windows (30–60 min) to accommodate variable infant sleep patterns.
Pharmacological treatment for insomnia during pregnancy and postpartum
Available guidelines and reviews for insomnia treatment include benzodiazepines and benzodiazepine-related drugs such as Z drugs, melatonin 2 mg prolonged release and melatonin receptor agonists, sedating antidepressants, and orexin receptor antagonists in the treatment of insomnia disorder (Sateia et al. 2017; Riemann et al. 2017; Frase et al. 2018; Palagini et al. 2020).
The National Institute for Health and Clinical Excellence (NICE) guideline on antenatal and postnatal mental health 2018 recommends that pharmacological treatment should be considered when women who do not respond to nonpharmacologic therapy and may present severe forms of insomnia symptoms, when there are no alternatives and the benefit outweighs the risk (Kay-Stacey and Attarian 2017). The US Food and Drug Administration (FDA) has categorized various drugs according to their risk during pregnancy and lactation (Howland 2009). However, in 2015, the FDA retired this system and ABCDX categories were replaced by the FDA Pregnancy and Lactation Labeling Rule (PLLR). New ruling provided prescribers with relevant information for critical decision-making reccomanding a shared decision-making approach when treating pregnant or lactating women an included three categories: (1) pregnancy, including labor and birth; (2) lactation; and (3) female and male subjects of reproductive potential (Watkins and Archambault 2016, Miller et al. 2020). Uguz (2021) proposed a safety scoring system for the use of psychotropic drugs during lactation based on the following 6 safety parameters: reported total sample, reported maximum relative infant dose, reported sample size for relative infant dose, infant plasma drug levels, prevalence of reported any adverse effect, and reported serious adverse effects. The total score ranges from 0 to 10. Higher scores represent a higher safety profile. Different meta-analyses and reviews discussed these issues related to insomnia treatment (Chaudhry and Susser 2018; Bei and Coo 2015; Miller et al. 2020; Uguz 2021
A recent meta-analysis showed that benzodiazepines and benzodiazepine-related drugs are commonly prescribed for the treatment of sleep problems and anxiety disorders during pregnancy with estimations of 27–93%, with a four times higher prevalence during pregnancy compared to the postpartum period; the prevalence seems highest in the third trimester (3.1%; CI 1.8−4.5%), followed by the first (0.5%; CI 0.3−0.7%), and second trimester (0.3%; CI 0.3−0.3%) (for an overview, see Bei and Coo 2015). Benzodiazepines and benzodiazepine-related drugs during pregnancy pass through the placenta, with a greater placental transfer in late pregnancy, compared to early pregnancy (Chaudhry and Susser 2018; Bei and Coo 2015). As reviewed from Bei and Coo 2015), the use of these drugs has been associated with a range of adverse birth outcomes including higher risk of spontaneous abortion (odds ratio (OR) 2.39, 95% confidence interval (CI) 2.10–2.73) (Sheehy et al. 2019) and preterm birth (OR 2.03, 95% CI 1.11–3.69) (Ogawa et al. 2018; Chaudhry and Susser 2018; Huitfeldt et al. 2020). Maternal use of benzodiazepines drugs in the third trimester has been associated with floppy infant syndrome, including symptoms of hypothermia, lethargy, and respiratory problems (Bulletins–Obstetrics 2008), and withdrawal symptoms which may persist for several months in the neonate (Bulletins–Obstetrics 2008). However, a meta-analysis in one million pregnancies did not find increased teratogenic risks, such as cardiovascular malformations and oral cleft, yielding an OR of 1.07 (95% CI 0.91–1.25) for cohort studies and of 1.27 (95% CI 0.69–2.32) for case-control studies (Enato et al. 2011). Indeed, Bais et al. (2020) observed that these studies on the use of benzodiazepines and benzodiazepine-related drugs during pregnancy remain therefore inconclusive; especially, the long-term effects are not entirely clear at this point (Bais et al. 2020).
In particular, the literature is not consistent in which trimester exposure would be more harmful for the fetus. On one hand, it is advised to avoid drug use during the first trimester, due to potential teratogenic risks, although these risks have thus far not been demonstrated by a meta-analysis (Bais et al. 2020). On the other hand, it is also mentioned that late third trimester use is associated with more risks for the fetus or neonate including the risk of floppy infant syndrome, which could lead to hypoxia and even irreversible damage in the neonate (Bulletins–Obstetrics 2008; Chaudhry and Susser 2018; Bais et al. 2020).
The most often used or prescribed benzodiazepine has been lorazepam. Lorazepam as other benzodiazepines showed positive evidence of human fetal risk, but potential benefits may warrant use of the drug in pregnant woman despite potential risk. Lorazepam is among the benzodiazepines most commonly prescribed during the lactation period (for an overview, see Uguz 2021). Lorazepam is a benzodiazepine with largest available data and in addition, no adverse effects in infants have been reported yet (Uguz 2021). However, almost a relative infant dose value of nearly 10% was reported in a patient and, additionally, the lack of data on infant plasma drug levels may confirm a potential moderate risk effect during lactation (Uguz 2021).
Among benzodiazepine-related drugs zolpidem, in animal reproduction, studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks. According to Uguz (2021), the lactation risk is high and it is not recommended for lactation. The most important restriction in the use of these drugs in lactating women is limited available data (Uguz 2021). Zopiclone has a moderate safety profile, and its usage during lactation is possible according to Uguz (2021).
For most other sedative-hypnotics, limited available data are available during pregnancy and postpartum; hence, they are not suggested during pregnancy and lactation.
About exogenous melatonin and melatonin receptor agonists, no human data are to date available during pregnancy and postpartum. Ramelteon melatonin receptor agonists are associated with teratogenicity in animal studies but no human data on either pregnancy or breastfeeding are available (Oyiengo et al. 2014; Miller et al. 2020). For these reasons, Ramelteon is not currently suggested for insomnia treatment during pregnancy and postpartum (Miller et al. 2020).
The effect of exogenous melatonin in pregnancy is not well studied, with conflicting results in mouse models (Miller et al. 2020). Although there are concerns regarding exogenous melatonin administration in pregnancy because it crosses the placenta and may have an impact on the development of circadian rhythms and reproductive function in the offspring, it may also have some potential fetal protective effects. On this topic, an ongoing trial is testing the neuroprotective effect of exogenous melatonin administration in fetuses diagnosed with growth restriction (Palmer et al. 2019). For these reasons, exogenous melatonin is not currently suggested for insomnia treatment during pregnancy and postpartum (Miller et al. 2020). In particular, since melatonin is often in over the counter formula, it is not suggested for insomnia treatment during pregnancy and postpartum since other substances which are not studied in pregnancy may be combined and included.
Although antihistamines are not recommended for insomnia treatment in the general population (Riemann et al. 2017; Palagini et al. 2020), they are widely used for insomnia treatment in pregnancy, in particular diphenhydramine (Miller et al. 2020). In addition, few studies confirm their safety profiles in humans and in particular, some of them reported various anomalies associated with the first trimester use (Kay-Stacey and Attarian 2017; Balserak and Lee 2017; Miller et al. 2020). No data are available for antihistamines use during lactation. Since antihistamines are not recommended for insomnia treatment and few human data are available for the treatment of insomnia during pregnancy and postpartum, their use may not be suggested for insomnia treatment during peripartum.
Among antidepressants, doxepin has been recommended for insomnia treatment (Riemann et al. 2017; Frase et al. 2018) and trazodone for insomnia treatment in patients over 65 years (Palagini et al. 2020). About doxepin, animal studies and human reports are both scarce in pregnancy (Miller et al. 2020) and it should be avoided during lactation (Uguz 2021). For this reason, the use of doxepin is not suggested for insomnia treatment during peripartum.
Data about bout trazodone could be promising, but they are limited. In animals at the highest dosage, trazodone was associated with a reduction in fetal viability in rats. In humans, no major congenital malformations have been reported based on few studies (McElhatton et al. 1996; Einarson et al. 2003; Einarson et al. 2009). The use of trazodone during lactation has been rated as possible with caution because limited data are available (Uguz 2021).
About orexin receptor antagonists which are approved for insomnia treatment in some countries (Sateia et al. 2017), there are some animal data about the use of suvorexant that reported no adverse fetal effects; indeed, there are no controlled data in human pregnancy. US FDA pregnancy category was C for suvorexant since there were not adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
Alternative therapies herbal or dietary supplements such as chamomile tea or lavender pillows acupuncture also are used as sleep aids but controlled studies are needed to assess the benefits and risks to fetal and maternal health (Bacaro et al. 2020) while mindfulness may be useful (Kalmbach et al. 2021a, 2021b).
Conclusions
Insomnia symptoms are frequent sleep disorders during pregnancy and postpartum and may be risk factors for perinatal psychopathology. Assessing and treating insomnia during peripartum period should be of importance and should be included in the routine evaluation of pregnant women; it may prevent peripartum psychopathology (Sharma et al. 2021). Evaluation of insomnia during peripartum may be conducted at least throughout clinical interview but also specific rating scales are available for peripartum period, which may help insomnia and sleep disturbances evaluation (Table 2). Although studies heterogeneity, the most used rating scale for insomnia evaluation during pregnancy was the Insomnia Severity Index (ISI). Future studies should include the use of ISI to evaluate and compare in different countries and races prevalence of insomnia during peripartum or the efficacy of this questionnaire in this population.
Cognitive behavioral therapy for insomnia (CBT-I) should be the preferred choice during peripartum for insomnia symptoms, as for insomnia patients. Indeed, some adaptations may be useful when treating insomnia for pre- or postpartum periods. Four studies proved that CBT-I administered in person via mail or digital approaches may be an effective treatment for insomnia during peripartum. CBT-I may also improve mood and anxiety symptoms, which can be correlated during pregnancy. Further studies are needed to better evaluate CBT-I efficacy in preventing peripartum psychopathology.
Pharmacological treatment may be considered when women who do not respond to nonpharmacologic therapy, hold severe forms of insomnia symptoms related to mood and anxiety disorders and when there are no alternatives and the benefits outweigh the risks (Table 3). A shared decision-making approach involving the mother and the family should be adopted when prescribing pharmacological therapy for insomnia during pregnancy.
Among the pharmacological options available for insomnia, limited data are available for pregnancy and lactation. Lorazepam has been the most studied compounds in pregnancy, and trazodone may be promising but to date limited data are available. Future observation is necessary to help managing pharmacological treatment of insomnia during peripartum.
References
ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists (2008) Use of psychiatric medications during pregnancy and lactation. ACOG Committee on Practice Bulletins--Obstetrics. Obstet Gynecol 111(4):1001–1020
Adler I, Weidner K, Eberhard-Gran M, Garthus-Niegel S (2021) The impact of maternal symptoms of perinatal insomnia on social-emotional child development: a population-based, 2-year follow-up study. Behav Sleep Med 19(3):303–317
American Psychiatric Association (2013) Sleep-wake disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA, American Psychiatric Association
Amezcua-Prieto C, Naveiro-Fuentes M, Arco-Jiménez N et al (2020) Walking in pregnancy and prevention of insomnia in third trimester using pedometers: study protocol of Walking_Preg project (WPP). A randomized controlled trial. BMC Pregnancy Childbirth 20(1):521
Anothaisintawee T, Reutrakul S, Van Cauter E et al (2016) Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med Rev. 30:11–24
Aukia L, Paavonen EJ, Jänkälä T, Tolvanen M, Korja R, Karlsson L, Karlsson H, Polo-Kantola P (2020) Insomnia symptoms increase during pregnancy, but no increase in sleepiness - associations with symptoms of depression and anxiety. Sleep Med 72:150-156
Bacaro V, Benz F, Pappaccogli A, De Bartolo P, Johann AF, Palagini L, Lombardo C, Feige B, Riemann D, Baglioni C (2020) Interventions for sleep problems during pregnancy: a systematic review. Sleep Med Rev 50:101234
Baglioni C, Palagini L (2021) CBT-I protocols for women’s age span. In: Baglioni C, Espie CA, Riemann D (eds) European CBT-I Textbook. Wiley and Sons, in press
Baglioni C, Tang NKY, Johann AF, Altena E, Bramante A, Riemann D, Palagini L (2020) Insomnia and poor sleep quality during peripartum: a family issue with potential long term consequences on mental health. J Matern Fetal Neonatal Med 2:1–9
Baglioni C, Bostanova Z, Bacaro V, Benz F, Hertenstein E, Spiegelhalder K, Rücker G, Frase L, Riemann D, Feige B (2020b) A systematic review and network meta-analysis of randomized controlled trials evaluating the evidence base of melatonin, light exposure, exercise, and complementary and alternative medicine for patients with insomnia disorder. J Clin Med 9(6):1949
Baglioni C, Altena E, Bjorvatn B et al (2020) The European Academy for Cognitive Behavioural Therapy for Insomnia: an initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J Sleep Res 29(2):12967
Bais B, Lindeboom R, van Ravesteyn L, Tulen J, Hoogendijk W, Lambregtse-van den Berg M, Kamperman A (2019) The impact of objective and subjective sleep parameters on depressive symptoms during pregnancy in women with a mental disorder: an explorative study. Int J Environ Res Public Health. 16(9):1587
Bais B, Molenaar NM, Bijma HH, Hoogendijk WJG, Mulder CL, Luik AI, Lambregtse-van den Berg MP, Kamperman AM (2020) Prevalence of benzodiazepines and benzodiazepine-related drugs exposure before, during and after pregnancy: a systematic review and meta-analysis. J Affect Disord. 269:18–27
Balserak BI, Lee KA (2017) Sleep and sleep disorders associated with pregnancy. Princ Pract Sleep Med 156:1525–1539
Bei B, Coo S (2015) Trinder sleep and mood during pregnancy and the postpartum period. Sleep Med Clin 10(1):25–33. https://doi.org/10.1016/j.jsmc.2014.11.011
Bei B, Pinnington DM, Shen L, Blumfield M, Drummond SPA, Newman LK, Manber R (2019) A scalable cognitive behavioural program to promote healthy sleep duringpregnancy and postpartum periods: protocol of a randomised controlled trial (the SEED project). BMC Pregnancy Childbirth 19(1):254
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28(2):193–213
Carney CE, Buysse DJ, Ancoli-Israel S, Edinger J, Krystal AD, Lichstein KL, Morin CM (2012) The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 35:287–302
Chaudhry SK, Susser LC (2018) Considerations in treating insomnia during pregnancy: a literature review. Psychosomatics. 59(4):341–348
Dorheim S, Bjorvatn B, Eberhard-Gran M (2012) Insomnia and depressive symptoms in late pregnancy: a population-based study. Behav Sleep Med 10(3):152–166
Drake C, Richardson G, Roehrs T, Scofield H, Roth T (2004) Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 27(2):285–291
Einarson A, Bonari L, Voyer-Lavigne S (2003) A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy. Can J Psychiatry 48(2):106–110
Einarson A, Choi J, Einarson TR, Koren G (2009) Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 54(4):242–246
Emamian F, Khazaie H, Okun ML et al (2019) Link between insomnia and perinatal depressive symptoms: a metaanalysis. J Sleep Res. 28(6):12858
Enato E, Moretti E, Koren G (2011) The fetal safety of benzodiazepines: an updated meta-analysis. J Obstet Gynaecol Can 33:46–48
Facco FL, Kramer JHo KH, Zee PC, Grobman WA (2010) Sleep disturbances in pregnancy. Obstet Gynecol 115:77–83
Felder J, Hartman A, Epel E, Prather A (2019) Pregnant patient perceptions of provider detection and treatment of insomnia. Behav Sleep Med 18(6):787–796
Felder JN, Epel ES, Neuhaus J, Krystal AD, Prather AA (2020) Efficacy of digital cognitive behavioral therapy for the treatment of insomnia symptoms among pregnant women: a randomized clinical trial. JAMA Psychiat 77(5):484–492
Fernández-Alonso AM, Trabalón-Pastor M, Chedraui P, Pérez-López FR (2012) Factors related to insomnia and sleepiness in the late third trimester of pregnancy. Arch Gynecol Obstet. 286(1):55–61
Frase L, Nissen C, Riemann D, Spiegelhalder K (2018) Making sleep easier: pharmacological interventions for insomnia. Expert Opin Pharmacother 19(13):1465–1473
Garbazza C, Hackethal S, Riccardi S et al (2020) Polysomnographic features of pregnancy: a systematic review. Sleep Med Rev. 50:101249
Gelaye B, Zhong QY, Barrios YV, Redline S, Drake CL, Williams MA (2016) Psychometric evaluation of the Ford Insomnia Response to Stress Test (FIRST) in early pregnancy. J Clin Sleep Med. 12(4):579–587
Gordon LK, Mason KA, Mepham E, Sharkey KM (2021) A mixed methods study of perinatal sleep and breastfeeding outcomes in women at risk for postpartum depression. Sleep Health. 7(3):353–361
Hertenstein E, Feige B, Gmeiner T et al (2019) Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 43:96–105
Wołyńczyk-Gmaj D, Różańska-Walędziak A, Ziemka S et al (2017) Insomnia in pregnancy is associated with depressive symptoms and eating at night. J Clin Sleep Med. 13(10):1171–1176
Howland RH (2009) Evaluating the safety of medications during pregnancy and lactation. J Psychosoc Nurs Ment Health Serv 47(3):19–22
Huang YJ, Ye Y, Huang XN, Feng WW, Chen Q, He CY, Li Z, Wang NR (2019) Association of maternal nocturnal sleep throughout pregnancy with the early nocturnal sleep of infants. 57(8):608-613
Huitfeldt A, Sundbakk LM, Skurtveit S, Handal M, Nordeng H (2020) Associations of maternal use of benzodiazepines or benzodiazepine-like hypnotics during pregnancy with immediate pregnancy outcomes in Norway. JAMA Netw Open 3(6):205860
Johns MW (1991) A new method of measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14:540
Juric S, Newport DJ, Ritchie JC, Galanti M, Stowe ZN (2009) Zolpidem (Ambien) in pregnancy: placental passage and outcome. Arch Womens Ment Health. 12(6):441–446
Kalmbach DA, Cheng P, Sangha R, O’Brien LM, Swanson LM, Palagini L, Bazan LF, Roth T, Drake CL (2019) Insomnia, short sleep, and snoring in mid-to-late pregnancy: disparities related to poverty, race, and obesity. Nat Sci Sleep. 11:301–315
Kalmbach DA, Cheng P, Ong JC et al (2020a) Depression and suicidal ideation in pregnancy: exploring relationships with insomnia, short sleep, and nocturnal rumination. Sleep Med 65:62-73
Kalmbach DA, Cheng P, O'Brien LM et al (2020b) A randomized controlled trial of digital cognitive behavioral therapy for insomnia in pregnant women. Sleep Med 72:82-92
Kalmbach DA, Roth T, Cheng P, Ong JC, Rosenbaum E, Drake CL (2020) Mindfulness and nocturnal rumination are independently associated with symptoms of insomnia and depression during pregnancy. Sleep Health 6(2):185–191
Kalmbach DA, Cheng P, Roth T, Swanson LM, Cuamatzi-Castelan A, Roth A, Drake CL (2021) Examining patient feedback and the role of cognitive arousal in treatment non-response to digital cognitive-behavioral therapy for insomnia during pregnancy. Behav Sleep Med. 15:1–20
Kalmbach DA, Cheng P, Drake CL (2021) A pathogenic cycle between insomnia and cognitive arousal fuels perinatal depression: exploring the roles of nocturnal cognitive arousal and perinatal-focused rumination. Sleep. 44(6):028
Kanto JH (1982) Use of benzodiazepines during pregnancy, labor and lactation, with particular reference to pharmacokinetic considerations. Drugs 23:354–380
Kantrowitz-Gordon I, McCurry SM, Landis CA, Lee R, Wi D (2020) Online prenatal trial in mindfulness sleep management (OPTIMISM): protocol for a pilot randomized controlled trial. Pilot Feasibility Stud. 6:128
Kay-Stacey M, Attarian HP (2017) Managing sleep disorders during pregnancy. Gender Genome 1:34–45
Kiviruusu O, Pietikäinen JT, Kylliäinen A, Pölkki P, Saarenpää-Heikkilä O, Marttunen M, Paunio T, Paavonen EJ (2020) Trajectories of mothers’ and fathers’ depressive symptoms from pregnancy to 24 months postpartum. J Affect Disord 260:629–637
Kızılırmak A, Timur S, Kartal B (2012) Insomnia in pregnancy and factors related toinsomnia. Sci World J 2012:197093
Ko H S, Shin J, Kim MY, Kim YH, Lee J, Kil KC, Moon HB, Lee G, Sa-Jin K, Kim B I (2012) Sleep disturbances in Korean pregnant and postpartum women. J Psychosom Obstet Gynecol 33(2):85–90
Kugbey N, Ayanore M, Doegah P, Chirwa M, Bartels SA, Davison CM, Purkey E (2021) Prevalence and correlates of prenatal depression, anxiety and suicidal behaviours in the Volta Region of Ghana. Int J Environ Res Public Health. 18(11):5857
Liset R, Grønli J, Henriksen RE, Henriksen TEG, Nilsen RM, Pallesen S (2021) Sleep, evening light exposure and perceived stress in healthy nulliparous women in the third trimester of pregnancy. PLoS ONE 16(6): 0252285
Louis JM, Koch MA, Reddy U M, Silver RM, Parker CB, Facco FL, Redline S, Nhan-Chan, C.L., Chung, JH, Pien GW, Basner RC, Grobman W A, Wing D A, Simhan H., Haas DM, Mercer B, Parry S, Mobley, D, Carper B, Zee PC (2018) Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol 218(5):1–12
Manber R, Steidtmann D, Chambers A, Ganger W, Horwitz S, Connelly C (2013) Factors associated with clinically significant insomnia among pregnant low-income Latinas. J Women’s Health 22(8):694–701
Manber R, Bei B, Simpson N, Asarnow L, Rangel E, Sit A, Lyell D (2019) Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 133(5):911–919
McElhatton PR, Garbis HM, Eléfant E (1996) The outcome of pregnancy in 689 women exposed to therapeutic doses of antidepressants. A collaborative study of the European Network of Teratology Information Services (ENTIS) Reproductive Toxicology 10(4):285–294
Meltzer LJ, Montgomery-Downs HE (2011) Sleep in the family. Pediatr Clin North Am. 58(3):765–774
Miller MA, Mehta N, Clark-Bilodeau C, Bourjeily G (2020) Sleep pharmacotherapy for common sleep disorders in pregnancy and lactation. Chest. 157(1):184–197
Mindell JA, Cook RA, Nikolovski J (2015) Sleep patterns and sleep disturbances across pregnancy. Sleep Med. 16(4):483–488
Mindell JA, Leichman ES, DuMond C et al (2017) Sleep and social-emotional development in infants and toddlers. J Clin Child Adolesc Psychol. 46(2):236–246
Moher D, Liberati A, Tetzlaff J et al (2009) The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 6:1000097
Morin CM (1993) Insomnia: psychological assessment and management. Guilford Press, New York
Mourady D, Richa S, Karam PT, Hajj Moussa F, El Osta N, Kesrouani A, Azouri J, Jabbour H, Hajj A, Rabbaa Khabbaz L (2017) Associations between quality of life, physical activity, worry, depression and insomnia: a cross-sectional designed study in healthy pregnant women. PLoS ONE 12(5)
Nacar G, Tashan S (2019) Relationship between sleep characteristics and depressive symptoms in last trimester of pregnancy. Afr Health Sci 19(4):2934–2944
National Institute for Health and Care Excellence NICE (2018) Antenatal and postnatal mental health clinical management and service guidance updated edition 2018
Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L (1985) The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther 23(3):263–271
Ogawa Y, Takeshima N, Furukawa TA (2018) Maternal exposure to benzodiazepine and risk of preterm birth and low birth weight: a case-control study using a claims database in Japan Asia Pac. Psychiatry 10:12309
Okun M, O’Brien L (2018) Concurrent insomnia and habitual snoring are associated with adverse pregnancy outcomes. Sleep Med 46:12–19
Okun ML, Schetter CD, Glynn LM (2011) Poor sleep quality is associated with preterm birth. Sleep. 34(11):1493–1498
Okun ML, Buysse DJ, Hall MH (2015) Identifying insomnia in early pregnancy: validation of the insomnia symptoms questionnaire (ISQ) in pregnant women. J Clin Sleep Med 11(6):645-54
Okun ML, Obetz V, Feliciano L (2021) Sleep disturbance in early pregnancy, but not inflammatory cytokines, may increase risk for adverse pregnancy outcomes. Int J Behav Med. 28(1):48–63
Osnes RS, Eberhard-Gran M, Follestad T, Kallestad H, Morken G, Roaldset JO (2020) Mid-pregnancy insomnia is associated with concurrent and postpartum maternal anxiety and obsessive-compulsive symptoms: a prospective cohort study. J Affect Disord. 266:319–326
Osnes RS, Eberhard-Gran M, Follestad T, Kallestad H, Morken G, Roaldset JO (2021) Mid-pregnancy insomnia and its association with perinatal depressive symptoms: a prospective cohort study. Behav Sleep Med. 19(3):285–302
Oyiengo D, Louis M, Hott B et al (2014) Sleep disorders in pregnancy. Clin Chest Med 35:571–587
Paine S-J, Signal TL, Sweeney B et al (2020) Maternal sleep disturbances in late pregnancy and the association with emergency caesarean section: a prospective cohort study. Sleep Health. 6(1):65–70
Palagini L, Maria Bruno R, Gemignani A et al (2013) Sleep loss and hypertension: a systematic review. Curr Pharm Des. 19(13):2409–2419
Palagini L, Gemignani A, Banti S, Manconi M, Mauri M, Riemann D (2014) Chronic sleep loss during pregnancy as a determinant of stress: impact on pregnancy outcome. Sleep Med. 15(8):853–859
Palagini L, Cipollone G, Masci I, Novi M, Caruso D, Kalmbach DA, Drake CL (2019) Stress-related sleep reactivity is associated with insomnia, psychopathology and suicidality in pregnant women: preliminary results. Sleep Med 56:145–150
Palagini L, Manni R, Aguglia E et al (2020) Expert opinions and consensus recommendations for the evaluation and management of insomnia in clinical practice: joint statements of five Italian scientific societies. Front Psychiatry. 11:558
Pallesen S, Bjorvatn B, Nordhus IH, Sivertsen B, Hjørnevik M, Morin CM (2008) A new scale for measuring insomnia: the Bergen Insomnia Scale. Percept Mot Skills. 107(3):691–706
Palmer KR, Mockler JC, Davies-Tuck ML (2019) Protect-me: a parallel-group, triple blinded, placebo-controlled randomised clinical trial protocol assessing antenatal maternal melatonin supplementation for fetal neuroprotection in early-onset fetal growth restriction. BMJ Open 9(6)
Partinen M, Gislason TJ (1995) Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. Sleep Res. 4(S1):150–155
Pengo MF, Won CH, Bourjeily G (2018) Sleep in women across the life span. Chest 154(1):196–206
Pietikäinen JT, Härkänen T, Polo-Kantola P et al (2021) Estimating the cumulative risk of postnatal depressive symptoms: the role of insomnia symptoms across pregnancy. Soc Psychiatry Psychiatr Epidemiol 56:2251–2261
Puertas-Gonzalez JA, Mariño-Narvaez C, Peralta-Ramirez MI, Romero-Gonzalez B (2021) The psychological impact of the COVID-19 pandemic on pregnant women. Psychiatry Res. 301:113978
Rezaei E, Moghadam ZB, Nejat S, Dehghannayeri N (2014) The impact of sleep healthy behavior education on the quality of life in the pregnant women with sleep disorder: a randomized control trial in the year 2012. Iran J Nurs Midwifery Res 19(5):508
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C (2010) The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev 14:19–31
Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K (2015) The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 14(5):547–558
Riemann D, Baglioni C, Bassett C et al (2017) European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 26:675–700
Román-Gálvez R, Amezcua-Prieto C, Salcedo-Bellido I, Martínez-Galiano J, Khan K, Bueno-Cavanillas A (2018) Factors associated with insomnia in pregnancy: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol 221:70–75
Ross LE, Murray BJ, Steiner M (2005) Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 30(4)
Sanchez SE, Friedman LE, Rondon MB, Drake CL, Williams MA, Gelaye B (2020) Association of stress-related sleep disturbance with psychiatric symptoms among pregnant women. Sleep Med. 70:27–32
Sedov ID, Tomfohr-Madsen LM (2021) Trajectories of insomnia symptoms and associations with mood and anxiety from early pregnancy to the postpartum. Behav Sleep Med. 19(3):395–340
Sedov ID, Goodman SH, Tomfohr-Madsen LM (2017) Insomnia treatment preferences during pregnancy. J Obstet Gynecol Neonat Nurs 46(3):95–104
Sedov I, Madsen J, Goodman S, Tomfohr-Madsen L (2018) Couples’ treatment preferences for insomnia experienced during pregnancy. Fam Syst Health 37(1):46–55
Sedov ID, Anderson NJ, Dhillon AK, Tomfohr-Madsen LM (2021) Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res 30(1):13207
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL (2017) Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 13(2):307–349
Sharma V, Mazmanian D (2003) Sleep loss and postpartum psychosis. Bipolar Disord 5:98–105
Sharma V, Palagini L, Riemann D (2021) Should we target insomnia to treat and prevent postpartum depression? J Matern Fetal Neonatal Med. 29:1–3
Sheehy O et al (2019) Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. J of the Am Med Assoc Psychiatry 76(9):948–957
Sivertsen B, Hysing M, Dørheim SK, Eberhard-Gran M (2015) Trajectories of maternal sleep problems before and after childbirth: a longitudinal population-based study. BMC Pregnancy Childbirth 15:129
Swanson LM, Kalmbach DA, Raglan GB, O'Brien LM (2020) Perinatal insomnia and mental health: a review of recent literature. Curr Psychiatry Rep. 2020;22(12):73
Sweeney BM, Signal TL, Babbage DR (2020) Effect of a behavioral-educational sleep intervention for first-time mothers and their infants: pilot of a controlled trial. J Clin Sleep Med. 16(8):1265–1274
Tikotzky L (2016) Postpartum maternal sleep, maternal depressive symptoms and self-perceived mother-infant emotional relationship. Behav Sleep Med. 14(1):5–22
Tinker S et al(2019) Use of benzodiazepine medications during pregnancy and potential risk for birth defects, national birth defects prevention study, 1997–2011. Birth Defects Res 111(10):613-620
Tomfohr LM, Buliga E, Letourneau NL et al (2015) Trajectories of sleep quality and associations with mood during the perinatal period. Sleep. 38(8):1237–1245
Tomfohr-Madsen LM, Clayborne ZM, Rouleau CR, Campbell TS (2017) Sleeping for two: an open-pilot study of cognitive behavioral therapy for insomnia in pregnancy. Behav Sleep Med. 15(5):377–393
Uguz FA (2021) New safety scoring system for the use of psychotropic drugs during lactation. Am J Ther. 28(1):118–120
Umeno S, Kato C, Nagaura Kondo H, Eto H (2020) Characteristics of sleep/wake problems and delivery outcomes among pregnant Japanese women without gestational complications. BMC Pregnancy Childbirth 20(1): 179
Wang LH, Lin HC, Lin CC, Chen YH, Lin HC (2010) Increased risk of adverse pregnancy outcomes in women receiving zolpidemduring pregnancy. Clin Pharmacol Ther. 88(3):369–374
Wang WJ, Hou CL, Jiang YP et al (2020) Prevalence and associated risk factors of insomnia among pregnant women in China. Compr Psychiatry 98:152168
Watkins EJ, Archambault M (2016) Understanding the new pregnancy and lactation drug labeling. JAAPA. 29(2):50–52
Funding
Open access funding provided by Università degli Studi di Ferrara within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Missing Open Access funding information has been added in the Funding Note.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palagini, L., Bramante, A., Baglioni, C. et al. Insomnia evaluation and treatment during peripartum: a joint position paper from the European Insomnia Network task force “Sleep and Women,” the Italian Marcè Society and international experts task force for perinatal mental health. Arch Womens Ment Health 25, 561–575 (2022). https://doi.org/10.1007/s00737-022-01226-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-022-01226-8