Abstract
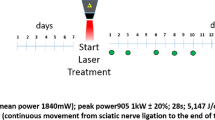
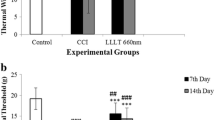
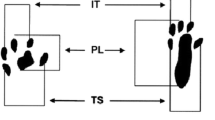
Neuropathic pain (NP) is one of the most suffered conditions in medical disciplines. The role of reactive oxygen species (ROS) and oxidative stress in the induction of NP was studied by many researchers. Neuropathies lead to medical, social, and economic isolation of the patient, so various therapies were used to treat or reduce it. During the recent years, low-level laser therapy (LLLT) has been used in certain areas of medicine and rehabilitation. Chronic constriction injury (CCI) is a well-known model for neuropathic pain studies. In order to find the effects of different wavelengths of LLLT on the injured sciatic nerve, the present research was done. Thirty Wistar adult male rats (230–320 g) were used in this study. The animals were randomly divided into three groups (n = 10). To induce neuropathic pain for the sciatic nerve, the CCI technique was used. Low-level laser of 660 and 980 nm was used for two consecutive weeks. Thermal and mechanical hyperalgesia was done before and after surgery on days 7 and 14, respectively. Paw withdrawal thresholds were also evaluated. CCI decreased the pain threshold, whereas both wavelengths of LLLT for 2 weeks increased mechanical and thermal threshold significantly. A comparison of the mechanical and thermal threshold showed a significant difference between the therapeutic effects of the two groups that received LLLT. Based on our findings, the laser with a 660-nm wavelength had better therapeutic effects than the laser with a 980-nm wavelength, so the former one may be used for clinical application in neuropathic cases; however, it needs more future studies.


Similar content being viewed by others
References
Merskey H, Bogduk N (1994) Part III: Pain terms, a current list with definitions and notes on usage. In: Merskey H, Bogduk N (eds) Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. IASP, Seattle, pp 209–214
WangLX WZJ (2003) Animal and cellular models of chronic pain. Adv Drug Deliv Rev 55:949–965
WoolfCJ MRJ (1999) Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353:1959–1964
Zimmermann M (2001) Pathobiology of neuropathic pain. Eur J Pharmacol 429:23–37
Dubner R (1991) Neuronal plasticity and pain following peripheral tissue inflammation or nerve injury. In: BondMR CJE, Woolf CJ (eds) Proceedings of the VIth World Congress on Pain. Elsevier, Amsterdam, pp 263–276
Woolf CJ (1996) Windup and central sensitization are not equivalent. Pain 66:105–108
Bennett GJ, Xie YK (1988) A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33:87–107
Gary J, Bennett G, Chung JM, Honore M, Seltzer Z (2003) Models of neuropathic pain in the rat. Current Protocols in Pharmacology. doi:10.1002/0471141755
Amour FE, Smith D (1941) A method for determining loss of pain sensation. J Pharmacol Exp Ther 72:74–79
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Espejo EF, Mir D (1993) Structure of the rat's behaviour in the hot plate test. Behav Brain Res 56:171–176
Woolfe G, Macdonald AD (1944) The evaluation of the anelgesic action of pethidine hydrochloride (Dermol). J Pharmacol Exp Ther 80:300–307
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J NeurosciMethods 53:55–63
Dubner R (1989) Methods of assessing pain in animals. In: Textbook of pain Edited by: Wall PD and Melzack R. Edinburgh, Churchville Livingstone: 247-256.
Mendonça AC, Barbieri CH, Mazzer N (2003) Directly applied low intensity direct electric current enhances peripheral nerve regeneration in rats. J Neurosci Methods 129:183–190. doi:10.1016/S0165-0270(03)00207-3
Gigo-Benato D, Geuna S, Rochkind S (2005) Phototherapy for enhancing peripheral nerve repair: a review of the literature. Muscle Nerve 31:694–701. doi:10.1002/mus.20305
Raso VVM, Barbieri CH, Mazzer N, Fasan VS (2005) Can therapeutic ultrasound influence the regeneration of the peripheral nerves. J Neurosci Methods 142:185–192
Ohshiro T, Calderhead RG (1988) Low-level laser therapy: a practical introduction. Wiley, New York, pp 17, 28–30, 33, 34.
Huang Y, Chen ACH, Carroll JD, Hamblin MR (2009) Biphasic dose response in low-level light therapy. Dose Response 7:358–383
Kitchen SS, Partridge CJ (1991) A review of low level laser therapy, part I: background, physiological effects and hazards. Physiotherapy 77:161–163
Karu TI, Pyatibrat L, Kalendo G (1995) Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27:219–233
Khullar SM, Brodin P, Fristad I, Kvinnsland IH (1999) Enhanced sensory reinnervation of dental target tissues in rats following low level laser (LLL) irradiation. Lasers Med Sci 14:177–184
Schindl A, Schindl M, Schindl L, Jurecka W, Hönigsmann H, Breier F (1999) Increased dermal angiogenesis after low-intensity laser therapy for a chronic radiation ulcer determined by a video measuring system. J Am AcadDermatol 40:481–484
Manteifel V, Bakeeva L, Karu T (1997) Ultrastructural changes in chondriome of human lymphocytes after irradiation with He-Ne laser: appearance of giant mitochondria. J Photochem Photobiol B 38:25–30
Reis FA, Belchior ACG, Carvalho PTC, Silva BAK, Pereira DM, Silva IS, Nicolau RA (2009) Effects of laser therapy (660 nm) on recovery of the sciatic nerve in rats after injury through neurotmesis followed by epineural anastomosis. Lasers Med Sci 24:741–747
Belchior ACG, Reis FA, Nicolau RA, Silva IS, Pereira DM, Carvalho PTC (2009) Influence of laser (660 nm) on functional recovery of the sciatic nerve in rats following crushing lesion. Lasers Med Sci 24:893–899
Barbosa RI, MarcolinoAM GRRJ, MazzerN BCH, Fonseca MCR (2010) Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med Sci 25:423–430
HsiehYL CLW, ChangPL YCC, KaoMJ HCZ (2012) Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1a (HIF-1a). The Journal of Comparative Neurology Research in Systems Neuroscience 520:2903–2916
Bertolini GR, Artifon EL, Silva TS, Cunha DM, Vigo PR (2011) Low-level laser therapy, at 830 nm, for pain reduction in experimental model of rats with sciatica. Arq Neuro Psiquiatr 69:356–359
RandallLO SJJ (1957) A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther 111:409–419
Cui JG, holmin S, mathiesen T (2000) Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 88(3):239–248
Martucci C, Trovato AE, Costa B, Borsani E, Franchi S, Magnaghi V, Panerai AE, Rodella LF, Valsecchi AE, Sacerdote P, Colleoni M (2008) The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 137:81–95
Sommer C, Kress M (2004) Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett 361:184–187
leung l, Cahill CM (2010) TNF-alpha and neuropathic pain--a review. J Neuroinflammation 7:27
Ferreira SH, Nakamura M, Abreu Castro MS (1978) The hyperalgesic effects of prostacyclin and prostaglandin E2. Prostaglandins 16(1):31–37
Higgs EA, Moncada S, Vane JR (1978) Inflammatory effects of prostacyclin (PGI2) and 6-oxo-PGF1[alpha] in the rat paw. Prostaglandins 16(2):153–162
Schepelmann K, Linger K, Schaibleh G (1992) Inflammatory mediators and nociception in the joint: excitation and sensitization of slowly conducting afferent fibers of cat's knee by prostaglandin I2. Neuroscience 50(1):237–247
DevorM WDM, Goetzl EJ (1992) Eicosanoids, but not tachykinins, excite C-fiber endings in rat sciatic nerve-end neuromas. Neuroreport 3(1):21–24
Cuzzocrea S, Thiemermann C, Salvemini D (2004) Potential therapeutic effect of antioxidant therapy in shock and inflammation. Curr Med Chem 11:1147–1162
Torres SH, De Sanctis JB, Briceño ML, Hernandez N, Finol H (2004) Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 181:419–427
Adams V, Nehrhoff B, Spate U, Linke A, Schulze PC, Baur A, Gielen S, Hambrecht R, Schuler G (2002) Induction of iNOS expression in skeletal muscle by IL-1b and NF-kB activation: An in vitro and in vivo study. Cardiovasc Res 54:95–104
Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J (2005) Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 15:113–120
Gilad E, Wong HR, Zingarelli B, Virag L, O'Connor M, Salzman AL, Szabo C (1998) Melatonin inhibits expression of the inducible isoform of nitric oxide synthase in murine macrophages: Role of inhibition of NFkappaB activation. FASEB J 12:685–693
RabeloSB VAB, NicolauR SMC, MeloMdaS PMT (2006) Comparison between wound healing in induced diabetic and nondiabetic rats after low-level laser therapy. Photomed Laser Surg 24(4):474–479
AlbertiniR AFS, CorreaFI RW, CogoJC AE, TeixeiraSA DNGHC, NetoCF ZRA, Lopes-Martins RA (2004) Effects of different protocol doses of low power gallium–aluminum–arsenate (Ga–Al–As) laser radiation (650 nm) on carrageenan induced rat paw oedema. J Photochem Photobiol B 74(2–3):101–107
FerreiraDM ZRA, VillaverdeAB CY, FrigoL PG, LongoI BDG (2005) Analgesic effect of He–Ne (632.8 nm) low-level laser therapy on acute inflammatory pain. Photomed Laser Surg 23(2):177–181
AlbertiniR VAB, AimbireF SMAC, BjordalJM ALP, MuninE CMS (2007) Anti-inflammatory effects of low-level laser therapy (LLLT) with two different red wavelengths (660 nm and 684 nm) in carrageenan-induced rat paw edema. J Photochem Photobiol B Biol 89:50–55
RizziCF, MaurizJL, Freitas CorreaDS, MoreiraAJ, ZettlerCG, FilippinLI, MarroniNP, Gonzalez-GallegoJ (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle, Lasers Surg. Med.
Moriyama Y, Moriyama EH, Blackmore K, Akens MK, Lilge L (2005) In vivo study of the inflammatory modulating effects of low level laser therapy on iNOS expression using bioluminescence imaging. Photochem Photobiol 81(6):1351–1355
Gavish L, Asher Y, Becker Y, Kleinman Y (2004) Low level laser irradiation stimulates mitochondrial membrane potential and disperses subnuclear promyelocytic leukemia protein. Lasers Surg Med 35(5):369–376
Gavish L, Perez L, Gertz SD (2006) Low-level laser irradiation modulates matrix metalloproteinase activity and gene expression in porcine aortic smooth muscle cells. Lasers Surg Med 38:779–786
Aimbire F, Albertini R, Pacheco MT, Castro-Faria-Neto HC, Leonardo PS, Iversen VV, Lopes-Martins RA, Bjordal JM (2006) Low-level laser therapy induces dose-dependent reduction of TNF alpha levels in acute inflammation. Photomed Laser Surg 24(1):33–37
Sakurai Y, Yamaguchi M, Abiko Y (2000) Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur J Oral Sci 108(1):29–34
Storz P (2007) Mitochondrial ROS—radical detoxification, mediated by protein kinase D. Trends Cell Biol 17:13–18
Brondon P, Stadler I, Lanzafame RJ (2005) A study of the effects of phototherapy dose interval on photobiomodulation of cell cultures. Lasers Surg Med 36:409–413
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B 49:1–17
Alexandratou E, Yova D, Handris P, Kletsas D, Loukas S (2002) Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy. PhotochemPhotobiolSci 1:547–552
Chen AC-H, Arany PR, Huang YY, Tomkinson EM, Saleem T, Yull FE, Blackwell TS, and Hamblin MR (2009) Low level laser therapy activates NF-κB via generation of reactive oxygen species in mouse embryonic fibroblasts. Proc SPIE in press.
Lavi R, Shainberg A, Friedmann H, Shneyvays V, Rickover O, Eichler M, Kaplan D, Lubart R (2003) Low energy visible light induces reactive oxygen species generation and stimulates anincrease of intracellular calcium concentration in cardiac cells. J Biol Chem 278:40917–40922
Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A (2005) Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 23:3–9
Pal G, Dutta A, Mitra K, Grace MS, Romanczyk TB, Wu X, Chakrabarti K, Anders J, Gorman E, Waynant RW, Tata DB (2007) Effect of low intensity laser interaction with human skin fibroblast cells using fiber-optic nano-probes. J Photochem Photobiol B 86:252–261
Zhang J, Xing D, Gao X (2008) Low-power laser irradiation activates Src tyrosine kinase through reactive oxygen species-mediated signaling pathway. J Cell Physiol 217:518–528
Wu Q, Huang YY, Dhital S, Hamblin MR, Anders JJ, Waynant RW (2010) Low level laser therapy for traumatic brain injury. Mechanisms for Low-Light Therapy V. Proc SPIE. 7552 Article No. 755206.
Amat A, Rigau J, Waynant RW, Ilev IK, Anders JJ (2006) The electric field induced by light can explain cellular responses to electromagnetic energy: a hypothesis of mechanism. J Photochem Photobiol B 82:152–160
Vladimirov Yu A In: Chikin S (1994) Efferent medicine. Institute of Biomedical Chemistry, Russian Academy of Medical Sciences, Moscow,pp.51–66.
Manteifel VM, Karu TI (2005) Structure of mitochondria and activity of their respiratory chain in successive generations of yeast cells exposed to He-Ne laser light. Izv Akad Nauk Ser Bioll 32:556–566
Hrnjak M, Kuljic-Kapulica N, Budisin A, Giser A (1995) Stimulatory effect of low-power density He-Ne laser radiation on human fibroblasts in vitro. Vojnosanit Pregl 52:539–546
Romm AR, Sherstnev MP, Volkov VV, Vladimirov Yu A (1986) The action of laser irradiation of peroxide chemiluminescence of wound exudation. Byul EkspBiol Med 102:426–428
Acknowledgements
The authors would like to thank the Medical Basic Sciences Laboratory (Faculty of Allied Medicine, TUMS) and Pain Laboratory (Faculty of Medicine, TUMS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masoumipoor, M., Jameie, S.B., Janzadeh, A. et al. Effects of 660- and 980-nm low-level laser therapy on neuropathic pain relief following chronic constriction injury in rat sciatic nerve. Lasers Med Sci 29, 1593–1598 (2014). https://doi.org/10.1007/s10103-014-1552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1552-1