Abstract
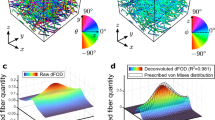
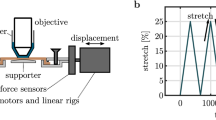
The affine transformation hypothesis is usually adopted in order to link the tissue scale with the fibers scale in structural constitutive models of fibrous tissues. Thanks to the recent advances in imaging techniques, such as multiphoton microscopy, the microstructural behavior and kinematics of fibrous tissues can now be monitored at different stretching within the same sample. Therefore, the validity of the affine hypothesis can be investigated. In this paper, the fiber reorientation predicted by the affine assumption is compared to experimental data obtained during mechanical tests on skin and liver capsule coupled with microstructural imaging using multiphoton microscopy. The values of local strains and the collagen fibers orientation measured at increasing loading levels are used to compute a theoretical estimation of the affine reorientation of collagen fibers. The experimentally measured reorientation of collagen fibers during loading could not be successfully reproduced with this simple affine model. It suggests that other phenomena occur in the stretching process of planar fibrous connective tissues, which should be included in structural constitutive modeling approaches.












Similar content being viewed by others
References
Alavi SH, Sinha A, Steward E et al (2015) Load-dependent extracellular matrix organization in atrioventricular heart valves: differences and similarities. Am J Physiol Heart Circ Physiol 309:H276–84. doi:10.1152/ajpheart.00164.2015
Bancelin S, Lynch B, Bonod-Bidaud C et al (2015) Ex vivo multiscale quantitation of skin biomechanics in wild-type and genetically-modified mice using multiphoton microscopy. Sci Rep 5:17635. doi:10.1038/srep17635
Benoit A, Latour G, Marie-Claire SK, Allain JM (2016) Simultaneous microstructural and mechanical characterization of human corneas at increasing pressure. J Mech Behav Biomed Mater 60:93–105. doi:10.1016/j.jmbbm.2015.12.031
Billiar KL, Sacks MS (1997) A method to quantify the fiber kinematics of planar tissues under biaxial stretch. J Biomech 30:753–756. doi:10.1016/S0021-9290(97)00019-5
Butler DL, Goldstein Sa, Guilak F (2001) Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res 122:S295–S305. doi:10.1115/1.1318906
Chandran PL, Barocas VH (2005) Affine versus non-affine fibril kinematics in collagen networks: theoretical studies of network behavior. J Biomech Eng 128:259. doi:10.1115/1.2165699
Chauvet D, Carpentier A, Allain J-M et al (2010) Histological and biomechanical study of dura mater applied to the technique of dura splitting decompression in Chiari type I malformation. Neurosurg Rev 33:287–294. doi:10.1007/s10143-010-0261-x (discussion 295)
Deyl Z, Macek K, Adam M, Vancíková O (1980) Studies on the chemical nature of elastin fluorescence. Biochim Biophys Acta 625:248–54
Fan R, Sacks MS (2014) Simulation of planar soft tissues using a structural constitutive model: finite element implementation and validation. J Biomech 47:2043–2054. doi:10.1016/j.jbiomech.2014.03.014
Fung YC (1990) Biomechanics: motion, flow, stress, and growth. Springer, New York
Gasser TC (2011) An irreversible constitutive model for fibrous soft biological tissue: a 3-D microfiber approach with demonstrative application to abdominal aortic aneurysms. Acta Biomater 7:2457–66. doi:10.1016/j.actbio.2011.02.015
Gasser TC, Ogden RW, Ga Holzapfel (2006) Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface R Soc 3:15–35. doi:10.1098/rsif.2005.0073
Gelse K, Pöschl E, Aigner T (2003) Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev 55:1531–1546. doi:10.1016/j.addr.2003.08.002
Goulam Houssen Y, Gusachenko I, Schanne-Klein M-C, Allain J-M (2011) Monitoring micrometer-scale collagen organization in rat-tail tendon upon mechanical strain using second harmonic microscopy. J Biomech 44:2047–2052. doi:10.1016/j.jbiomech.2011.05.009
Hannafin JA, Arnoczky SP (1994) Effect of cyclic and static tensile loading on water content and solute diffusion in canine flexor tendons: an in vitro study. J Orthop Res 12:350–6. doi:10.1002/jor.1100120307
Holzapfel GA, Stadler M, Schulze-Bauer CAJ (2002) A layer-specific three-dimensional model for the simulation of balloon angioplasty using magnetic resonance imaging and mechanical testing. Ann Biomed Eng 30:753–767. doi:10.1114/1.1492812
Humphrey JD (2003) Review paper: continuum biomechanics of soft biological tissues. Proc R Soc A Math Phys Eng Sci 459:3–46. doi:10.1098/rspa.2002.1060
Jayyosi C (2015) Caractérisation mécanique et microstructurale du comportement à rupture de la capsule de Glisson pour la prédiction du risque de lésions des tissus hépatiques humains. Université Claude Bernard Lyon 1
Jayyosi C, Coret M, Bruyère-Garnier K (2016) Characterizing liver capsule microstructure via in situ bulge test coupled with multiphoton imaging. J Mech Behav Biomed Mater 54:229–243. doi:10.1016/j.jmbbm.2015.09.031
Jayyosi C, Fargier G, Coret M, Bruyère-Garnier K (2014) Photobleaching as a tool to measure the local strain field in fibrous membranes of connective tissues. Acta Biomater 10:2591–601. doi:10.1016/j.actbio.2014.02.031
Keyes JT, Lockwood DR, Simon BR, Vande Geest JP (2013) Deformationally dependent fluid transport properties of porcine coronary arteries based on location in the coronary vasculature. J Mech Behav Biomed Mater 17:296–306. doi:10.1016/j.jmbbm.2012.10.002
Lanir Y, Salant EL, Foux A (1988) Physico-chemical and microstructural changes in collagen fiber bundles following stretch in-vitro. Biorheology 25:591–603
Loerakker S, Ristori T, Baaijens FPT (2016) A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves. J Mech Behav Biomed Mater 58:173–187. doi:10.1016/j.jmbbm.2015.10.001
Lynch B, Bancelin S, Bonod-Bidaud C et al (2016) A novel microstructural interpretation for the biomechanics of mouse skin derived from multiscale characterization. Acta Biomater. doi:10.1016/j.actbio.2016.12.051
Mauri a, Perrini M, Mateos JM (2013) Second harmonic generation microscopy of fetal membranes under deformation: normal and altered morphology. Placenta 34:1020–1026. doi:10.1016/j.placenta.2013.09.002
Mauri A, Ehret AE, Perrini M et al (2015) Deformation mechanisms of Human amnion: quantitative studies based on second harmonic generation microscopy. J Biomech. doi:10.1016/j.jbiomech.2015.01.045
Obbink-Huizer C, Oomens CWJ, Loerakker S et al (2014) Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech Model Mechanobiol 13:227–236. doi:10.1007/s10237-013-0501-4
Puxkandl R, Zizak I, Paris O et al (2002) Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci 357:191–197. doi:10.1098/rstb.2001.1033
Ramanujan S (1914) Modular equations and approximations to pi. Q J Math 45:350–372
Raub CB, Unruh J, Suresh V et al (2008) Image correlation spectroscopy of multiphoton images correlates with collagen mechanical properties. Biophys J 94:2361–2373. doi:10.1529/biophysj.107.120006
Rezakhaniha R, Agianniotis a, Schrauwen JTC et al (2012) Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol 11:461–473. doi:10.1007/s10237-011-0325-z
Robert L (2002) Elastin, past, present and future. Pathol Biol 50:503–511. doi:10.1016/S0369-8114(02)00336-X
Sacks MS (2003) Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J Biomech Eng 125:280. doi:10.1115/1.1544508
Screen HRC, Evans SL (2009) Measuring strain distributions in tendon using confocal microscopy and finite elements. J Strain Anal Eng Des 44:327–335. doi:10.1243/03093247JSA491
Tang T, Ebacher V, Cripton P et al (2015) Shear deformation and fracture of human cortical bone. Bone 71:25–35. doi:10.1016/j.bone.2014.10.001
Vijayaraghavan S, Huq R, Hausman MR (2014) Methods of peripheral nerve tissue preparation for second harmonic generation imaging of collagen fibers. Methods 66:246–255. doi:10.1016/j.ymeth.2013.08.012
Voss B, Rauterberg J, Allam S, Pott G (1980) Distribution of collagen type I and type III and of two collagenous components of basement membranes in the human liver. Pathol Res Pract 170:50–60. doi:10.1016/S0344-0338(80)80155-5
Zoumi A, Lu X, Kassab GS, Tromberg BJ (2004) Imaging coronary artery microstructure using second-harmonic and two-photon fluorescence microscopy. Biophys J 87:2778–2786. doi:10.1529/biophysj.104.042887
Zoumi A, Yeh A, Tromberg BJ (2002) Imaging cells and extracellular matrix in vivo by using second-harmonic generation and two-photon excited fluorescence. Proc Natl Acad Sci USA 99:11014–11019. doi:10.1073/pnas.172368799
Acknowledgements
The authors wish to thank Pr Mathias Brieu for useful discussions. This work was supported by the Programme Avenir Lyon Saint-Etienne (ANR-11-IDEX-0007) of Université de Lyon, within the program “Investissements d’Avenir” operated by the French National Research Agency (ANR), and by grants from Ecole Polytechnique (interdisciplinary project) and from Agence Nationale de la Recherche (ANR-13-BS09-0004-02 and ANR-10-INBS-04).
Conflict of interest
None of the authors have any professional or financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
J-M. Allain, K. Bruyère-Garnier and M. Coret have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jayyosi, C., Affagard, JS., Ducourthial, G. et al. Affine kinematics in planar fibrous connective tissues: an experimental investigation. Biomech Model Mechanobiol 16, 1459–1473 (2017). https://doi.org/10.1007/s10237-017-0899-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0899-1