Abstract
Objective
Magnetic resonance imaging (MRI) of the lung remains challenging due to the low tissue density, susceptibility artefacts, unfavourable relaxation times and motion. Previously, we demonstrated in vivo that one-lung flooding (OLF) with saline is a viable and safe approach. This study investigates the feasibility of OLF in an MRI environment and evaluates the flooding process on MR images.
Methods
OLF of the left lung was performed on five animals using a porcine model. Before, during and after OLF, standard T2w and T1w spin-echo (SE) and gradient-echo (GRE) sequences were applied at 3 T.
Results
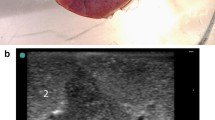
The procedure was successfully performed in all animals. On T1w MRI, the flooded lung appeared homogenous and isointense with muscle tissue. On T2w images, vascular structures were highly hypointense, while the bronchi were clearly demarcated with hypointense wall and hyperintense lumen. The anatomical demarcation of the flooded lung from the surrounding organs was superior on T2w images. No outflow effects were seen, and no respiration triggering was required.
Discussion
OLF can be safely performed in an MR scanner with highly detailed visualization of the pulmonary structures on T2w images. The method provides new approaches to MRI-based image-guided pulmonary interventions using the presented experimental model.






Similar content being viewed by others
Abbreviations
- CT:
-
Computed tomography
- ECG:
-
Electrocardiogram
- EPI:
-
Echo planar imaging
- FA:
-
Flip angle
- GRE:
-
Gradient echo
- HASTE:
-
Half-fourier acquisition single-shot turbo spin echo
- KCl:
-
Potassium chloride
- mAP:
-
Mean arterial pressure
- MRI:
-
Magnetic resonance imaging
- MRgFUS:
-
Magnetic resonance-guided focused ultrasound surgery
- OLF:
-
One-lung flooding
- PFC:
-
Perfluorocarbon
- PFH:
-
Perfluorohexane
- CO2 :
-
Carbon dioxide partial pressure
- pO2 :
-
Oxygen partial pressure
- PFC:
-
Perfluorocarbon liquids
- ROI:
-
Region of interest
- SAR:
-
Specific absorption rate
- SE:
-
Spin echo
- SO2 :
-
Oxygen saturation
- T:
-
Tesla
- TE:
-
Echo time
- TR:
-
Repetition time
References
Wild J, Marshall H, Bock M, Schad L, Jakob P, Puderbach M, Molinari F, Van Beek E, Biederer J (2012) MRI of the lung (1–3). Insights Imaging 3(4):345–353
Biederer J, Beer M, Hirsch W, Wild J, Fabel M, Puderbach M, Van Beek E (2012) MRI of the lung (2/3). Why … when … how? Insights Imaging 3(4):355–371
Wielpütz M, Kauczor H (2012) MRI of the lung: state of the art. Diagn Interv Radiol 18(4):344–353
Kurihara Y, Matsuoka S, Yamashiro T, Fujikawa A, Matsushita S, Yagihashi K, Nakajima Y (2014) MRI of pulmonary nodules. Am J Roentgenol AJR 202(3):210–216
Renz D, Scholz O, Böttcher J, Maurer M, Denecke T, Schwarz C, Pfeil A, Streitparth F, Huppertz A, Mehl A, Poellinger A, Staab D, Hamm B, Mentzel H (2015) Comparison between magnetic resonance imaging and computed tomography of the lung in patients with cystic fibrosis with regard to clinical, laboratory, and pulmonary functional parameters. Invest Radiol 50(10):733–742
Rieke V, Butts-Pauly K (2008) MR thermometry. J Magn Reson Imaging 27(2):376–390
Sprinkhuizen S, Konings M, van der Bom M, Viergever M, Bakker C, Bartels L (2010) Temperature-induced tissue susceptibility changes lead to significant temperature errors in PRFS-based MR thermometry during thermal interventions. Magn Reson Med 64(5):1360–1372
Miller G, Altes T, Brookeman J, De Lange E, Mugler J (2004) Hyperpolarized 3He lung ventilation imaging with B1-inhomogeneity correction in a single breath-hold scan. MAGMA 16(5):218–226
Kjørstad Å, Regier M, Fiehler J, Sedlacik J (2017) A decade of lung expansion: a review of ventilation-weighted 1H lung MRI. Z Med Phys 27(3):172–179
Obruchkov S, Noseworthy M (2016) (1)H-MR imaging of the lungs at 3.0 T. Quant Imaging Med Surg 6(1):67–75
Herrmann K, Krämer M, Reichenbach J (2016) Time efficient 3D radial UTE sampling with fully automatic delay compensation on a clinical 3 T MR scanner. PLoS One 11(3):e0150371
Gibiino F, Sacolick L, Menini A, Landini L, Wiesinger F (2015) Free-breathing, zero-TE MR lung imaging. MAGMA 28(3):207–215
Oechsner M, Pracht E, Staeb D, Arnold J, Köstler H, Hahn D, Beer M, Jakob P (2009) Lung imaging under free-breathing conditions. Magn Reson Med 61(3):723–727
Khrapitchev A, Larkin J, Melemenidis S, Papoutsis K, Thelwall P, Sibson N (2015) Perfluorohexane liquid MRI of mouse lungs in a dual-tuned 1H/19F coil. ISMRM, Toronto
Wolfson M, Shaffer T (2005) Pulmonary applications of perflourchemical liquids: ventilation and beyond. Pediatric Resp Rev 6:117–127
Weigel J, Steinmann D, Emerich P, Stahl C, v Elverfeldt D, Guttmann J (2001) High-resolution three-dimensional 19F-magnetic resonance imaging of rat lung in situ: evaluation of airway strain in the perfluorocarbon-filled lung. Physiol Meas 32(2):251–262
Chenoune M, De Rochefort L, Bruneval P, Lidouren F, Kohlhauer M, Seemann A, Ghaleh B, Korn M, Dubuisson R, Ben Yahmed A, Maître X, Isabey D, Ricard J, Kerber R, Darrasse L, Berdeaux A, Tissier R (2014) Evaluation of lung recovery after static administration of three different perfluorocarbons in pigs. BMC Pharmacol Toxicol 15:53
Lesser T, Klinzing S, Schubert H, Klein U, Bartel M (1998) Lung flooding–a new method for complete lung sonography. Res Exp Med 198(2):83–91
Klinzing S, Lesser T, Schubert H, Bartel M, Klein U (2000) One lung flooding for video-assisted thoracoscopic surgery in animal experiments on pigs. Resp Exp Med 199:333–337
Wolfram F, Boltze C, Schubert H, Bischoff S, Lesser T (2014) Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur J Med Res 19:1
Elster A (1993) Sellar susceptibility artifacts: theory and implications. AJNR 14:129–136
Klinzing S, Lesser T, Schubert H, Bloos F, Klein U, Bartel M (1999) Hemodynamics and gas exchange during experimental one-lung fluid flooding in pigs. Res Exp Med 199(2):87–94
Klinzing S, Lesser T, Schubert H, Bartel M, Klein U (2001) Wet to Dry ratio of lung tissue and surfactant outwash after one lung flooding. Eur Journ Med Res 200(1):27–33
Lesser T, Klinzing S, Schubert H, Kosmehl M (2008) Consequences of one-lung flooding: a histological and immunological investigation. Eur J Med Res 13(9):432–438
Kiorpes A, MacWilliams P, Schenkman DI, Bäckström L (1990) Blood gas and hematological changes in experimental peracute porcine pleuropneumonia. Can J Vet Res 54(1):164
Wolfram F, Reichenbach J, Lesser T (2013) An ex vivo human lung model for ultrasound guided HIFU therapy using lung flooding. Ultrasound Med Biol 40(3):496–503
Lesser T, Schubert H, Bischoff S, Wolfram F (2013) Lung flooding enables efficient lung sonography and tumour imaging in human ex vivo and porcine in vivo lung cancer models. Eur J Med Res 18:23
Stafford R, Fuentes D, Elliott A, Weinberg J, Ahrar K (2010) Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng 38(1):79–100
Jolesz F, Hynynen K, McDannold N, Tempany C (2005) MR imaging-controlled focused ultrasound ablation: a noninvasive image-guided surgery. Magn Reson Imaging Clin North Am 13(3):545–560
Yuan J, Mei C, Panych L, McDonnald N, Madore B (2012) Torwards fast and accurate temperature mapping with proton resonance frequency based MR thermometry. Quant Imaging Med Surg 2(1):21–32
Yeow K, See L, Lui K, Lin M, Tsao T, Ng K, Liu H (2001) Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 12(11):1305–1312
Komlosi P, Altes T, Qing K, Mooney K, Miller G, Mata J, de Lange E, Tobias W, Cates G, Brookeman J, Mugler J (2015) Regional anisotropy of airspace orientation in the lung as assessed with hyperpolarized helium-3 diffusion MRI. J Magn Reson Imaging 42(6):1777–1782
Schmidt R, Lang F (2007) Human Physiology, 30th edn. Springer, Berlin
McGee K, Mariappan Y, Hubmayr R, Carter R, Bao Z, Levin D, Manduca A, Ehman R (2012) Magnetic resonance assessment of parenchymal elasticity in normal and edematous, ventilator-injured lung. J Appl Physiol 113(4):666
Appleby C, Towner R (2001) Magnetic resonance imaging of pulmonary damage in the term and premature rat neonate exposed to hyperoxia. Pediatr Res 50(4):502–507
Lesser T, Schubert H, Güllmar D, Reichenbach J, Wolfram F (2016) One-lung flooding reduces the ipsilateral diaphragm motion during mechanical ventilation. Eur J Med Res 21:9
Author information
Authors and Affiliations
Contributions
FW, TGL and DG developed the study design and conception. Güllmar and Wolfram performed acquisition of MR data and statistics; HS, SB, and TGL performed care of animal and vital monitoring during OLF; JB, JRR and FW performed analysis and interpreting of data; JRR, JB, TGL and FW were drafting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
This research was supported by the Focused Ultrasound Surgery Foundation, Charlottesville, VA, USA (FUS325) and the SRH Waldklinikum Gera, Germany. The authors declare that they have no competing interests, neither financial nor non-financial.
Ethical standards
All national guidelines for care and use of animals were followed. Animal experiments were performed with permission from the Veterinary Department of the Thuringian State Authority for Food Protection and Fair Trading (TLLV Reg15-003/12) in compliance with the National Animal Protection Act.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wolfram, F., Güllmar, D., Böttcher, J. et al. Assessment of MR imaging during one-lung flooding in a large animal model. Magn Reson Mater Phy 32, 581–590 (2019). https://doi.org/10.1007/s10334-019-00759-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-019-00759-x