Abstract
Purpose
Neurotensin receptor-1 (NTS1) is increasingly recognized as a potential target in diverse tumors including breast cancer, but factors associated with NTS1 expression have not been fully clarified.
Methods
We studied NTS1 expression using the Tissue MicroArray (TMA) of primary breast tumors from Institut Bergonié. We also studied association between NTS1 expression and clinical, pathological, and biological parameters, as well as patient outcomes.
Results
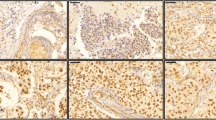
Out of 1419 primary breast tumors, moderate to strong positivity for NTS1 (≥ 10% of tumoral cells stained) was seen in 459 samples (32.4%). NTS1 staining was cytoplasmic in 304 tumors and nuclear in 155 tumors, a distribution which appeared mutually exclusive. Cytoplasmic overexpression of NTS1 was present in 21.5% of all breast tumors. In multivariate analysis, factors associated with cytoplasmic overexpression of NTS1 in breast cancer samples were higher tumor grade, Ki67 ≥ 20%, and higher pT stage. Cytoplasmic NTS1 was more frequent in tumors other than luminal A (30% versus 17.3%; p < 0.0001). Contrastingly, the main “correlates” of a nuclear location of NTS1 were estrogen receptor (ER) positivity, low E&E (Elston and Ellis) grade, Ki67 < 20%, and lower pT stage. In NTS1-positive samples, cytoplasmic expression of NTS1 was associated with shorter 10-year metastasis-free interval (p = 0.033) compared to NTS1 nuclear staining. Ancillary analysis showed NTS1 expression in 73% of invaded lymph nodes from NTS1-positive primaries.
Conclusion
NTS1 overexpression was found in about one-third of breast tumors from patients undergoing primary surgery with two distinct patterns of distribution, cytoplasmic distribution being more frequent in aggressive subtypes. These findings encourage the development of NTS1-targeting strategy, including radiopharmaceuticals for imaging and therapy.




Similar content being viewed by others
References
Morgat C, Mishra AK, Varshney R et al (2014) Targeting Neuropeptide Receptors for Cancer Imaging and Therapy: Perspectives with Bombesin, Neurotensin, and Neuropeptide-Y Receptors. J Nucl Med 55:1650–1657. https://doi.org/10.2967/jnumed.114.142000
Younes M, Wu Z, Dupouy S et al (2014) Neurotensin (NTS) and its receptor (NTSR1) causes EGFR, HER2 and HER3 over-expression and their autocrine/paracrine activation in lung tumors, confirming responsiveness to erlotinib. Oncotarget 5:8252–8269
Ye Y, Long X, Zhang L et al (2016) NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 7:70303–70322. https://doi.org/10.18632/oncotarget.11854
Zhang Y, Zhu S, Yi L et al (2014) Neurotensin receptor1 antagonist SR48692 reduces proliferation by inducing apoptosis and cell cycle arrest in melanoma cells. Mol Cell Biochem 389:1–8. https://doi.org/10.1007/s11010-013-1920-3
Körner M, Waser B, Strobel O et al (2015) Neurotensin receptors in pancreatic ductal carcinomas. EJNMMI Res. https://doi.org/10.1186/s13550-015-0094-2
Agopiantz M, Forgez P, Casse J-M et al (2017) Expression of neurotensin receptor 1 in endometrial adenocarcinoma is correlated with histological grade and clinical outcome. Virchows Arch 471:521–530. https://doi.org/10.1007/s00428-017-2215-y
Zhou Z, Zhou Z, Xie J et al (2015) The significance of NTR1 expression and its correlation with β-catenin and EGFR in gastric cancer. Diagn Pathol 10:128. https://doi.org/10.1186/s13000-015-0356-3
Dupouy S, Viardot-Foucault V, Alifano M et al (2009) The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS One 4:e4223. https://doi.org/10.1371/journal.pone.0004223
Dupouy S, Doan VK, Wu Z et al (2014) Activation of EGFR, HER2 and HER3 by neurotensin/neurotensin receptor 1 renders breast tumors aggressive yet highly responsive to lapatinib and metformin in mice. Oncotarget 5:8235–8251
Sauerbrei W, Taube SE, McShane LM et al (2018) Reporting Recommendations for tumor marker prognostic studies (REMARK): An abridged explanation and elaboration. J Natl Cancer Inst 110:803–811. https://doi.org/10.1093/jnci/djy088
Morgat C, Macgrogan G, Brouste V et al (2017) Expression of gastrin-releasing peptide receptor in breast cancer and its association with pathologic, biologic, and clinical parameters: A study of 1,432 primary tumors. J Nucl Med 58:1401–1407
Reubi JC (2014) Strict rules are needed for validation of G-protein-coupled receptor immunohistochemical studies in human tissues. Endocrine 47:659–661. https://doi.org/10.1007/s12020-014-0320-0
Riehle KJ, Kenerson HL, Riggle KM et al (2019) Neurotensin as a source of cyclic AMP and co-mitogen in fibrolamellar hepatocellular carcinoma. Oncotarget 10:5092–5102. https://doi.org/10.18632/oncotarget.27149
Souazé F, Dupouy S, Viardot-Foucault V et al (2006) Expression of neurotensin and NT1 receptor in human breast cancer: A potential role in tumor progression. Cancer Res 66:6243–6249. https://doi.org/10.1158/0008-5472.CAN-06-0450
Morgat C, Chastel A, Molinie V et al (2019) Neurotensin receptor-1 expression in human prostate cancer: A pilot study on primary tumors and lymph node metastases. Int J Mol Sci 20:1721. https://doi.org/10.3390/ijms20071721
Cochrane DE, Carraway RE, Harrington K et al (2011) HMC-1 human mast cells synthesize neurotensin (NT) precursor, secrete bioactive NT-like peptide(s) and express NT receptor NTS1. Inflamm Res 60:1139–1151. https://doi.org/10.1007/s00011-011-0378-6
Allison KH, Hammond MEH, Dowsett M et al (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. https://doi.org/10.1200/JCO.19.02309
Chastel A, Worm DJ, Alves ID et al (2020) Design, synthesis, and biological evaluation of a multifunctional neuropeptide-Y conjugate for selective nuclear delivery of radiolanthanides. EJNMMI Res 10:16. https://doi.org/10.1186/s13550-020-0612-8
Bird JL, Simpson R, Vllasaliu D, Goddard AD (2017) Neurotensin receptor 1 facilitates intracellular and transepithelial delivery of macromolecules. Eur J Pharm Biopharm 119:300–309. https://doi.org/10.1016/j.ejpb.2017.06.027
Rodríguez Y, Almeida TA, Valladares F et al (2010) Neurotensin and neurotensin receptor 1 expression in human myometrium and uterine leiomyomas. Biol Reprod 83:641–647. https://doi.org/10.1095/biolreprod.110.084962
Toy-Miou-Leong M, Cortes CL, Beaudet A et al (2004) Receptor trafficking via the perinuclear recycling compartment accompanied by cell division is necessary for permanent neurotensin cell sensitization and leads to chronic mitogen-activated protein kinase activation. J Biol Chem 279:12636–12646. https://doi.org/10.1074/jbc.M303384200
Toy-Miou-Leong M, Bachelet C-M, Pélaprat D et al (2004) NT agonist regulates expression of nuclear high-affinity neurotensin receptors. J Histochem Cytochem 52:335–345. https://doi.org/10.1177/002215540405200304
Feldberg RS, Cochrane DE, Carraway RE et al (1998) Evidence for a neurotensin receptor in rat serosal mast cells. Inflamm res 47:245–250. https://doi.org/10.1007/s000110050325
Hwang JR, Baek MW, Sim J et al (2010) Intermolecular cross-talk between NTR1 and NTR2 neurotensin receptor promotes intracellular sequestration and functional inhibition of NTR1 receptors. Biochem Biophys Res Commun 391:1007–1013. https://doi.org/10.1016/j.bbrc.2009.12.007
He T, Wang M, Wang H et al (2019) Evaluation of neurotensin receptor 1 as potential biomarker for prostate cancer theranostic use. Eur J Nucl Med Mol Imaging 46:2199–2207. https://doi.org/10.1007/s00259-019-04355-y
Gromova P, Rubin BP, Thys A et al (2011) Neurotensin receptor 1 Is expressed in gastrointestinal stromal tumors but not in interstitial cells of cajal. PLoS One 6:e14710. https://doi.org/10.1371/journal.pone.0014710
Zhang X, Fan S, Zhang L, Shi Y (2020) Glucagon-like peptide-1 receptor undergoes importin-α-dependent nuclear localization in rat aortic smooth muscle cells. FEBS Lett 594:1506–1516. https://doi.org/10.1002/1873-3468.13751
Müller C, Umbricht CA, Gracheva N et al (2019) Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-019-04345-0
Hindié E, Zanotti-Fregonara P, Quinto MA et al (2016) Dose deposits from 90Y, 177Lu, 111In, and 161Tb in micrometastases of various Sizes: implications for radiopharmaceutical therapy. J Nucl Med 57:759–764
Alcocer-Ávila ME, Ferreira A, Quinto MA et al (2020) Radiation doses from 161Tb and 177Lu in single tumour cells and micrometastases. EJNMMI Physics 7:33. https://doi.org/10.1186/s40658-020-00301-2
Callegari CCF, Cavalli IJ, Lima RS et al (2016) Copy number and expression analysis of FOSL1, GSTP1, NTSR1, FADD and CCND1 genes in primary breast tumors with axillary lymph node metastasis. Cancer Genet 209:331–339. https://doi.org/10.1016/j.cancergen.2016.06.003
Baum RP, Singh A, Schuchardt C et al (2018) 177Lu-3BP-227 for neurotensin receptor 1–targeted therapy of metastatic pancreatic adenocarcinoma: first clinical results. J Nucl Med 59:809–814. https://doi.org/10.2967/jnumed.117.193847
Maschauer S, Einsiedel J, Hübner H et al (2016) 18F- and 68Ga-labeled neurotensin peptides for PET imaging of neurotensin receptor 1. J Med Chem 59:6480–6492. https://doi.org/10.1021/acs.jmedchem.6b00675
Feng H, Zhang H, Wang M et al (2020) Improving tumor-to-background contrast through hydrophilic tetrazines: The construction of 18f labeled pet agents targeting non-small cell lung carcinoma. Chemistry 26:4690–4694. https://doi.org/10.1002/chem.202000028
Fanelli R, Chastel A, Previti S et al (2020) Silicon-containing neurotensin analogues as radiopharmaceuticals for NTS1-positive tumors imaging. Bioconjugate Chem 31:2339–2349. https://doi.org/10.1021/acs.bioconjchem.0c00419
Groheux D, Hindie E (2021) Breast cancer: initial workup and staging with FDG PET/CT. Clin Transl Imaging 9:221–231. https://doi.org/10.1007/s40336-021-00426-z
Chan DL, Pavlakis N, Schembri GP et al (2017) Dual somatostatin Receptor/FDG PET/CT imaging in metastatic neuroendocrine tumours: proposal for a novel grading scheme with prognostic significance. Theranostics 7:1149–1158. https://doi.org/10.7150/thno.18068
Hofman MS, Emmett L, Sandhu S et al (2021) [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397:797–804. https://doi.org/10.1016/S0140-6736(21)00237-3
Funding
This work was funded by Institut National du Cancer (INCa PLBIO 2017, THERACAN project) and was achieved within the context of the Laboratory of Excellence TRAIL ANR-10-LABX-57.
Author information
Authors and Affiliations
Contributions
CM contributes to the conception, acquisition, analysis, interpretation of data, and funding and wrote the manuscript. VB analyzed and interpreted the data and approved the final version of the manuscript. AC contributes to data acquisition and approved the final version of the manuscript. VV acquired the data and approved the final version of the manuscript. GMG acquired and analyzed the data and approved the final version of the manuscript. EH analyzed and interpreted the data, participated in the funding, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of Institut Bergonié (Breast Research Group) who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the IRB of Institut Bergonié.
Informed consent
The Ethics committee of Institut Bergonié waived requirement for informed consent for the present retrospective analysis.
Cell lines
MCF-7, MDA-MB-453, MDA-MB-468, SKBR3, T47D, and ZR75.1 cell lines were obtained from Dr N. Jones (Univ. Bordeaux, France) and no additional authentication was performed by the authors of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Morgat, C., Brouste, V., Chastel, A. et al. Expression of neurotensin receptor-1 (NTS1) in primary breast tumors, cellular distribution, and association with clinical and biological factors. Breast Cancer Res Treat 190, 403–413 (2021). https://doi.org/10.1007/s10549-021-06402-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06402-5