Abstract
Background
The impact of short or prolonged use of triple therapy (TT) on outcomes in patients with atrial fibrillation (AF) and high risk of bleeding undergoing percutaneous coronary intervention (PCI) is unclear. We compared clinical outcomes according to the duration of TT in patients with AF and HAS-BLED ≥ 3 at 1 year of follow-up.
Methods
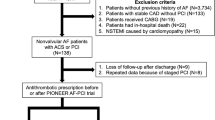
A prospective observational cohort enrolled 735 patients with AF between 2010 and 2015. Of these, 521 (70.9%) had HAS-BLED ≥ 3 and 380 (72.9%) were discharged on TT. TT was prescribed for 1 month in 233 patients (61.3%). The primary endpoint was the incidence of Bleeding Academic Research Consortium (BARC ≥ 3). The secondary endpoint was the occurrence of ischemic events (cardiac death, MI, stroke, or stent thrombosis).
Results
Patients on 1-month TT had a higher median HAS-BLED. Intracraneal hemorrhage was twofold more frequently in patients on > 1-month TT but without statistical significance (0.9% vs 2.1%, p = 0.20). Rates of the primary endpoint (bleeding BARC ≥ 3) were 8.2% vs 10.9% and did not differ between groups, while secondary endpoint did not occur more frequently in the 1-month TT group compared with the > 1-month TT group (26.6% vs 23.1%). In adjusted multivariate analyses, patients receiving 1-month TT had a similar risk of the primary endpoint compared to those with > 1-month TT (HR 1.47; 95% CI 0.48–4.47, p = 0.50). No difference was found in the secondary ischemic endpoint (HR 1.24; 95% CI 0.77–2.00, p = 0.38).
Conclusions
In patients with AF undergoing PCI at lower ischemic risk and higher bleeding risk, 1 month of TT seems safe and efficacious. Further studies are warranted in patients at high ischemic risk.


Similar content being viewed by others
References
Kralev S, Schneider K, Lang S, Suselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first-time coronary angiography. PLoS One. 2011;6:e24964.
Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–60.
Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective-2018 update. Circulation. 2018;138:527–36.
Ruiz-Nodar JM, Marín F, Roldán V, Valencia J, Manzano-Fernández S, Caballero L, et al. Should we recommend oral anticoagulation therapy in patients with atrial fibrillation undergoing coronary artery stenting with a high HAS-BLED bleeding risk score? Circ Cardiovasc Interv. 2012;5:459–66.
Romaguera R, Wakabayashi K, Laynez-Carnicero A, Sardi G, Maluenda G, Ben-Dor I, et al. Association between bleeding severity and long-term mortality in patients experiencing vascular complications after percutaneous coronary intervention. Am J Cardiol. 2012;109:75–81.
Sambola A, Ferreira-González I, Angel J, Alfonso F, Maristany J, Rodríguez O, et al. Therapeutic strategies after coronary stenting in chronically anticoagulated patients: the MUSICA study. Heart. 2009;18:1483–8.
Sørensen R, Hansen ML, Abildstrom SZ, Hvelplund A, Andersson C, Jørgensen C, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374:1967–74.
Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 126:1185–93.
Sambola A, Mutuberría M, García Del Blanco B, Alonso A, Barrabés JA, Alfonso F, et al. Effects of Triple therapy in patients with non-valvular atrial fibrillation undergoing percutaneous coronary intervention regarding thromboembolic risk stratification. Circ J. 2016;80:354–62.
Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433–41.
Sambola A, Mutuberría M, García Del Blanco B, Alonso A, Barrabés JA, Bueno H, et al. Impact of Triple therapy in elderly patients with atrial fibrillation undergoing percutaneous coronary intervention. PLoS One. 2016;11:e0147245.
European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, et al. Guidelines for the management of atrial fibrillation: the Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;19:2369–429.
Fiedler KA, Maeng M, Mehilli J, Schulz-Schüpke S, Byrne RA, Sibbing D, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE trial. J Am Coll Cardiol. 2015;65:1619–29.
Koskinas KC, Raber L, Zanchin T, Pilgrim T, Stortecky S, Hunziker L, et al. Duration of triple therapy antithrombotic therapy and outcomes among patients undergoing percutaneous coronary intervention. JACC Cardiovascular Interv. 2016;9:1473–83.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35.
Cutlip DE, Windecker S, Mehran R, Academic Research Consortium. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51.
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–47.
Ruiz-Nodar JM, Marín F, Sánchez-Payá J, Hurtado JA, Valencia-Martín J, Manzano-Fernández S, et al. Efficacy and safety of drug-eluting stent use in patients with atrial fibrillation. Eur Heart J. 2009;30:932–9.
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165.
Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–15.
Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–34.
Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. RE-DUAL PCI. Dual Antithrombotic Therapy With Dabigatran After Pci In Atrial Fibrillation. N Engl J Med. 2017;377:1513–24.
Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–24.
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–66.
Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, et al. ZEUS Investigators, Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805–15.
Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, et al. SENIOR investigators, drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. 2018;391:41–50.
Bonaca MP, Braunwald E, Sabatine MS. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–800.
Raccah BH, Perlman A, Danenberg HD, Pollak A, Muszkat M, Matok I. Major bleeding and hemorrhagic stroke with direct oral anticoagulants in patients with renal failure: systematic review and metaanalysis of randomized trials. Chest. 2016;149:1516–24.
Funding
This study was supported by CIBERCV. No additional external funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Sambola reports grant from Amgen to the institution, fees from lectures, Amgen, Boehringer-Ingelheim, Bristol-Myers, BMS-Pfizer, Astra-Zeneca, Novartis, Novo-nordisk outside the submitted work. Dr. Bueno reports grants from Instituto de Salud Carlos III; personal fees from Bayer; personal fees from Novartis; grants, personal fees, and non-financial support from AstraZeneca; grants and personal fees from BMS-Pfizer; personal fees from Ferrer; personal fees from MEDSCAPE-the Heart-org; and personal fees from Janssen, outside the submitted work. The remaining authors no have disclosures to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Triple therapy during 1 month is safe and efficacious for patients with AF and high risk of bleeding undergoing PCI.
• Bleeding and ischemic events were similar between patients treated on 1-month TT compared with those treated more than 1 month. However, the incidence of endocraneal hemorrhage was lower in patients on short-term TT.
• Triple therapy during 1 month was preferentially used in those at lower ischemic risk and higher bleeding risk.
• One month of TT should be reasonable as duration of treatment for patients with AF at low ischemic risk and high risk of bleeding undergoing PCI.
Electronic supplementary material
Supplementary Table 1
(DOCX 13 kb)
Supplementary Table 2
(DOC 49 kb)
Rights and permissions
About this article
Cite this article
Sambola, A., Bueno, H., Miranda, B. et al. Safe and Efficacious Use of 1-Month Triple Therapy in Patients with Atrial Fibrillation and High Bleeding Risk Undergoing PCI. Cardiovasc Drugs Ther 33, 425–433 (2019). https://doi.org/10.1007/s10557-019-06889-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-019-06889-7