Abstract
Purpose
The in-vitro environment influences oocyte competence and gene expression in cumulus cells and oocytes. Effects of culturing under non-attachment conditions and varying follicle exposure to FSH were investigated at the mRNA level and on oocyte developmental capacity.
Methods
Quantitative PCR analysis of Gdf9, Mater, Nmp2 (in oocytes), Lhcgr and Amh (in cumulus cells), and oocyte developmental competence after in vitro follicle culture were evaluated.
Results
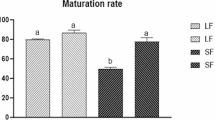
Follicle survival (98.7%) and polar body rate (94%) were similar for all conditions. Estradiol and progesterone production were significantly lower in non-attachment follicles (10-fold and 3-fold, respectively). Under non-attachment conditions, a higher two-cell rate (69.9%) and total blastocyst yield (48.5%) were obtained and, by decreasing FSH levels during culture, Lhcgr transcripts were significantly reduced to levels similar to in-vivo. Levels of oocyte-specific transcripts were not significantly influenced by in-vitro conditions.
Conclusion
Non-attachment conditions influence follicle steroid secretory capacity and, together with dynamic FSH doses, positively influence cumulus cell gene expression and oocyte developmental competence.




Similar content being viewed by others
References
Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197–207.
Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–66.
O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod. 2003;68:1682–6.
Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414.
Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75.
Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123:185–202.
Kim DH, Ko DS, Lee HC, Lee HJ, Park WI, Kim SS, et al. Comparison of maturation, fertilization, development, and gene expression of mouse oocytes grown in vitro and in vivo. J Assist Reprod Genet. 2004;21:233–40.
Combelles CMH, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20:1349–58.
Eppig JJ, O’Brien MJ, Wigglesworth K, Nicholson A, Zhang W, King BA. Effect of in vitro maturation oocytes on the health of adult offspring. Hum Reprod. 2009;24:922–8.
Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–53.
Latham KE, Bautista FDM, Hirao Y, O’Brien MJ, Eppig JJ. Comparison of protein synthesis patterns in mouse cumulus cells and mural granulosa cells: effects of follicle-stimulating hormone and insulin on granulosa cell differentiation in vitro. Biol Reprod. 1999;61:482–92.
Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163:109–16.
Sánchez F, Adriaenssens T, Romero S, Smitz J. Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod. 2010;83:514–24.
Sánchez F, Romero S, Smitz J. Oocyte and cumulus cell transcripts from cultured mouse follicles are induced to deviate from normal in vivo condition by combinations of insulin, follicle-stimulating hormone, and human chorionic gonadotropin. Biol Reprod. 2011;85:565–74.
Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349–62.
Mitchell LM, Kennedy CR, Hartshorne GM. Effects of varying gonadotropin dose and timing on antrum formation and ovulation efficiency of mouse follicles in vitro. Hum Reprod. 2002;17:1181–8.
Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408.
Nayudu PL, Fehrenbach A, Kiesel P, Vitt UA, Pancharatna K, Osborn S. Progress toward understanding follicle development in vitro: appearances are not deceiving. Arch Med Res. 2001;32:587–94.
Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod. 1994;9:527–32.
Rose UM, Hanssen RG, Kloosterboer HJ. Development and characterization of an in vitro ovulation model using mouse ovarian follicles. Biol Reprod. 1999;61:503–11.
Vitt UA, Nayudu PL, Rose UM, Kloosterboer HJ. Embryonic development after follicle culture is influenced by follicle-stimulating hormone isoelectric point range. Biol Reprod. 2001;65:1542–7.
Bishonga C, Takahashi Y, Katagiri S, Nagano M, Ishikawa A. In vitro growth of mouse ovarian preantral follicles and the capacity of their oocytes to develop to the blastocyst stage. J Vet Med Sci. 2001;63:619–24.
Pangas SA, Saudye H, Shea L, Woodruff T. Novel approach for the three-dimensional culture of granulosa cell–oocyte complexes. Tissue Eng. 2003;9:1013–21.
Xu M, West E, Shea LD, Woodruff TK. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–23.
West-Farrell E, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80:432–9.
Cortvrindt R, Smitz J. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243–54.
Romero S, Sánchez F, Adriaenssens T, Smitz J. Mouse cumulus-oocyte complexes from in vitro-cultured preantral follicles suggest an anti-luteinizing role for the EGF cascade in the cumulus cells. Biol Reprod. 2011;84:1164–70.
Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48:798–806.
Segers I, Adriaenssens T, Ozturk E, Smitz J. Acquisition and loss of oocyte meiotic and developmental competence during in vitro antral follicle growth in mouse. Fertil Steril. 2010;93:2695–700.
Romero S, Smitz J. Improvement of in vitro culture of mouse cumulus–oocyte complexes using PDE3-inhibitor followed by meiosis induction with epiregulin. Fertil Steril. 2010;93:936–44.
Convery M, Brawer JR. Thecal and interstitial cells in polycystic ovaries (PCO) in the rat. Anat Rec. 1991;231:324–32.
Lenie S, Smitz J. Estrogen receptor subtypes localization shifts in cultured mouse ovarian follicles. Histochem Cell Biol. 2008;129:827–40.
Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–72.
Zuccotti M, Piccinelli A, Giorgi Rossi P, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41:479–85.
Zuccotti M, Giorgi Rossi P, Martinez A, Garagna S, Forabosco A, Redi CA. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod. 1998;58:700–4.
Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60:580–7.
De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol. 2006;296:1–12.
Fehrenbach A, Nüsse N, Nayudu PL. Patterns of growth, oestradiol and progesterone released by in vitro cultured mouse ovarian follicles indicate consecutive selective events during follicle development. J Reprod Fertil. 1998;113:287–97.
Vitt UA, Kloosterboer HJ, Rose UM, Mulders JW, Kiesel PS, Bete S, et al. Isoforms of human recombinant follicle-stimulating hormone: comparison of effects on murine follicle development in vitro. Biol Reprod. 1998;59:854–61.
Rowghani NM, Heise MK, McKeel D, McGee EA, Koepsel RR, Russell AJ. Maintenance of morphology and growth of ovarian follicles in suspension culture. Tissue Eng. 2004;10:545–52.
Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–23.
Wu J, Nayudu PL, Kiesel PS, Michelmann HW. Luteinizing hormone has a stage-limited effect on preantral follicle development in vitro. Biol Reprod. 2000;63:320–7.
Gomes JE, Correia SC, Gouveia-Oliveira A, Cidadão AJ, Plancha CE. Three-dimensional environments preserve extracellular matrix compartments of ovarian follicles and increase FSH-dependent growth. Mol Reprod Dev. 1999;54:163–72.
Hillier SG, Whitelaw PF, Smyth CD. Follicular oestrogen synthesis: the ‘two-cell, two-gonadotrophin’ model revisited. Mol Cell Endocrinol. 1994;100:51–4.
Carabatsos MJ, Elvin J, Matzuk MM, Albertini DF. Characterization of oocyte and follicle development in growth differentiation factor-9-deficient mice. Dev Biol. 1998;204:373–84.
Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–48.
Vitt UA, Hayashi M, Klein C, Hsueh AJW. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–7.
Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82–83:431–46.
Tong ZB, Gold L, Pfeifer KE, Dorward H, Lee E, Bondy CA, et al. Mater, a maternal effect gene required for early embryonic development in mice. Nature Genet. 2000;26:267–740.
Burns KH, Viveiros MM, Ren Y, Wang P, DeMayo FJ, Frail DE, et al. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science. 2003;300:633–6.
Inoue A, Aoki F. Role of the nucleoplasmin 2 C-terminal domain in the formation of nucleolus-like bodies in mouse oocytes. FASEB J. 2010;24:485–94.
Baarends WM, Uilenbroek JTJ, Kramer P, Hoogerbrugge JW, van Leeuwen ECM, Themmen APN, et al. Anti-Müllerian hormone and anti-Müllerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinol. 1995;136:4951–62.
Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266:201–8.
Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–40.
Grøndahl ML, Nielsen ME, Dal Canto MB, Fadini R, Rasmussen IA, Westergaard LG, et al. Anti-Müllerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod Biomed Online. 2011;22:389–98.
Acknowledgements
The authors wish to acknowledge Mrs. Katy Billooye for her valuable technical assistance with staining for alkaline phosphatase in theca cells and for performing the radioimmunoassays on the conditioned media. The authors are very grateful to Ms. Sandra De Schaepdryver for her editorial support.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Financial support
This work was supported by The Belgian Foundation Against Cancer (project no. 221.2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Flor Sánchez and Sergio Romero contributed equally to this work.
Capsule
Non-attached follicle culture and decreased FSH levels modulate follicle steroidogenesis and positively influence gene expression in cumulus cells and oocyte developmental capacity.
Rights and permissions
About this article
Cite this article
Sánchez, F., Romero, S., Albuz, F.K. et al. In vitro follicle growth under non-attachment conditions and decreased FSH levels reduces Lhcgr expression in cumulus cells and promotes oocyte developmental competence. J Assist Reprod Genet 29, 141–152 (2012). https://doi.org/10.1007/s10815-011-9690-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-011-9690-x