Abstract
Purpose
We evaluated the relationship between meiotic spindle characteristics and in vitro fertilization cycle outcome.
Methods
Five hundred sixty-nine oocytes from 86 in vitro fertilization cycles were analyzed for fertilization and subsequent implantation rates. Oocytes were assessed for maturation status. The oocytes and embryos were cultured in sequential and nonsequential media (G Series, Vitrolife, Sweden) and incubated in 6% CO2, 5% O2 at 37 °C.
Two hours following oocyte decumulation (38–39 h post-hCG/GnRH administration) and prior to microinjection, the structure of the meiotic spindle was assessed using the Oosight Imaging System (CRI, UK).
Results
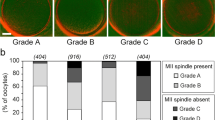
Four hundred fifty-six oocytes (80.5%) had a visible meiotic spindle, 82 (14.7%) had no meiotic spindle, and 31 (5.5%) were in telophase I. Oocytes exhibiting a meiotic spindle had a significantly higher fertilization rate and a lower rate of abnormal fertilization. Implantation data were obtained for 195 of the embryos transferred. The implantation rate for embryos derived from oocytes with a meiotic spindle was 32.9%, while in embryos originating from oocytes without a meiotic spindle and oocytes in telophase, this value dropped significantly (8.8 and 0%, respectively). To determine the correlation between retardance values and implantation rate for each oocyte, we established four groups, finding a range of retardance values with significantly higher implantation rates (27.5, 21, 29.3, and 53.8%, respectively).
Conclusion
Meiotic spindle imaging may be a valuable tool for prediction of oocyte quality, and retardance values of meiotic spindles, together with classical morphological classification, can be useful to select embryos with a higher implantation potential.



Similar content being viewed by others
References
Grady R, Alavi N, Vale R, Khandwala M, McDonald SD. Elective single embryo transfer and perinatal outcomes: a systematic review and meta-analysis. Fertil Steril. 2012;97:324–31.
Pinborg A. IVF/ICSI twin pregnancies: risks and prevention. Hum Reprod Update. 2005;11:575–93.
Workshop Group TEC. Multiple gestation pregnancy. Hum Reprod. 2000;15:1856–64.
Balaban B, Urman B, Ata B, Isiklar a, Larman MG, Hamilton R, et al. A randomized controlled study of human day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–82.
Ku PY, Lee RKK, Lin SY, Lin MH, Hwu YM. Comparison of the clinical outcomes between fresh blastocyst and vitrified-thawed blastocyst transfer. J Assist Reprod Genet. 2012;29:1353–6.
Roque M, Lattes K, Serra S, Sola I, Geber S, Carreras R, et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156–62.
Basirat Z, Rad HA, Esmailzadeh S, Jorsaraei SGA, Hajian-Tilaki K, Pasha H, et al. Comparison of pregnancy rate between fresh embryo transfers and frozen-thawed embryo transfers following icsi treatment. Iran J Reprod Med. 2016;14:39–46.
Gomaa H, Baydoun R, Sachak S, Lapana I, Soliman S. Elective single embryo transfer: is frozen better than fresh? J Bras Reprod Assist. 2016;20:3–7.
Vaiarelli A, Cimadomo D, Capalbo A, Orlando G, Sapienza F, Colamaria S, et al. Pre-implantation genetic testing in ART: who will benefit and what is the evidence? J Assist Reprod Genet. 2016;33:1273–8.
Krisher RL, Schoolcraft WB, Katz-Jaffe MG. Omics as a window to view embryo viability. Fertil Steril. 2015;103:333–41.
Montag M, Toth B, Strowitzki T. New approaches to embryo selection. Reprod BioMed Online. 2013;27:539–46.
Murakoshi Y, Sueoka K, Takahashi K, Sato S, Sakurai T, Tajima H, et al. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J Assist Reprod Genet. 2013;30:1367–75.
Kovacs P. Embryo selection: the role of time-lapse monitoring. Reprod Biol Endocrinol. 2014;12:124.
Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–77.
Rienzi L, Vajta G, Ubaldi F. Predictive value of oocyte morphology in human IVF: a systematic review of the literature. Hum Reprod Update. 2011;17:34–45.
Howe K, Fitz HG. Recent insights into spindle function in mammalian oocytes and early embryos. Biol Reprod. 2013;89:71.
Wang Z-B, Schatten H, Sun Q-Y. Why is chromosome segregation error in oocytes increased with maternal aging? Physiology. 2011;26:314–25.
Aman RR, Parks JE. Effects of cooling and rewarming on the meiotic spindle and chromosomes of in vitro-matured bovine oocytes. Biol Reprod. 1994;50:103–10.
Wang WH, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by Polscope before insemination. Hum Reprod. 2001a;16:1464–8.
Wang WH, Meng L, Hackett RJ, Odenbourg R, Keefe DL. The spindle observation and its relationship with fertilization after intracytoplasmic sperm injection in living human oocytes. Fertil Steril. 2001b;75:348–53.
Braga DPDAF, Figueira RDCS, Rodrigues D, Madaschi C, Pasqualotto FF, Iaconelli A, et al. Prognostic value of meiotic spindle imaging on fertilization rate and embryo development in in vitro-matured human oocytes. Fertil Steril. 2008;90:429–33.
Cohen Y, Malcov M, Schwartz T, Mey-Raz N, Carmon A, Cohen T, et al. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19:649–54.
Konc J, Kanyó K, Cseh S. Visualization and examination of the meiotic spindle in human oocytes with polscope. J Assist Reprod Genet. 2004;21:349–53.
Madaschi C, de Souza Bonetti TC, de Almeida Ferreira Braga DP, Pasqualotto FF, Iaconelli A, Borges E. Spindle imaging: a marker for embryo development and implantation. Fertil Steril. 2008;90:194–8.
Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18:817–20.
Rama Raju G a, Prakash GJ, Krishna KM, Madan K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: a preliminary study using PolScope imaging. Reprod BioMed Online. 2007;14:166–74.
Shen Y, Stalf T, Mehnert C, De Santis L, Cino I, Tinneberg H-R, et al. Light retardance by human oocyte spindle is positively related to pronuclear score after ICSI. Reprod BioMed Online. 2006;12:737–51.
Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, et al. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod BioMed Online. 2006;13:661–7.
Fang C, Tang M, Li T, Peng WL, Zhou CQ, Zhuang GL, et al. Visualization of meiotic spindle and subsequent embryonic development in in vitro and in vivo matured human oocytes. J Assist Reprod Genet. 2007;24:547–51.
Kilani S, Cooke S, Kan A, Chapman M. Are there non-invasive markers in human oocytes that can predict pregnancy outcome? Reprod BioMed Online. 2009;18:674–80.
Jose delos Santos M, Arroyo G, Busquet A, Calderon G, Cuadros J, Hurtado de Mendoza MV, et al. A multicenter prospective study to assess the effect of early cleavage on embryo quality, implantation, and live-birth rate. Fertil Steril. 2014;101:981–7.
Rienzi L, Ubaldi F, Martinez F, Iacobelli M, Minasi MG, Ferrero S, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18:1289–93.
Montag M, van der Ven H. Symposium: innovative techniques in human embryo viability assessment. Oocyte assessment and embryo viability prediction: birefringence imaging. Reprod BioMed Online. 2008;17:454–60.
Heindryckx B, De Gheselle S, Lierman S, Gerris J, De Sutter P. Efficiency of polarized microscopy as a predictive tool for human oocyte quality. Hum Reprod. 2011;26:535–44.
Shen Y, Betzendahl I, Tinneberg HR, Eichenlaub-Ritter U. Enhanced polarizing microscopy as a new tool in aneuploidy research in oocytes. Mutat Res - Genet Toxicol Environ Mutagen. 2008;651:131–40.
Montag M, Schimming T, van der Ven H. Spindle imaging in human oocytes: the impact of the meiotic cell cycle. Reprod BioMed Online. 2006;12:442–6.
Rienzi L, Ubaldi F, Iacobelli M, Minasi MG, Romano S, Greco E. Meiotic spindle visualization in living human oocytes. Reprod BioMed Online. 2005;10:192–8.
Wang WH, Keefe DL. Prediction of chromosome misalignment among in vitro matured human oocytes by spindle imaging with the PolScope. Fertil Steril. 2002;78:1077–81.
Woodward BJ, Montgomery SJ, Hartshorne GM, Campbell KH, Kennedy R. Spindle position assessment prior to ICSI does not benefit fertilization or early embryo quality. Reprod BioMed Online. 2008;16:232–8.
Taylor TH, Chang C, Elliott T, Colturato LF, Kort HI, Peter Z. Effect of denuding on polar body position in in- vitro matured oocytes. Reprod BioMed Online. 2008;17:515–9.
Korkmaz C, Sakinci M, Bayoglu Tekin Y, Ercan CM. Do quantitative birefringence characteristics of meiotic spindle and zona pellucida have an impact on implantation in single embryo transfer cycles? Arch Gynecol Obstet. 2014;289:433–8.
Montag M, Köster M, van der Ven K, van der Ven H. Gamete competence assessment by polarizing optics in assisted reproduction. Hum Reprod Update. 2011;17:654–66.
Liu L, Trimarchi JR, Oldenbourg R, Keefe DL. Increased birefringence in the meiotic spindle provides a new marker for the onset of activation in living oocytes. Biol Reprod. 2000;63:251–8.
Navarro P, Liu L, Trimarchi J, Ferriani R, Keefe D. Noninvasive imaging of spindle dynamics during mammalian oocyte activation. Fertil Steril. 2005;83:1197–205.
Sun X-F, Zhang W-H, Chen X-J, Xiao G-H, Mai W-Y, Wang W-H. Spindle dynamics in living mouse oocytes during meiotic maturation, ageing, cooling and overheating: a study by polarized light microscopy. Zygote. 2004;12:241–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Oro, S., Rey, M.I., Rodríguez, M. et al. Predictive value of spindle retardance in embryo implantation rate. J Assist Reprod Genet 34, 617–625 (2017). https://doi.org/10.1007/s10815-017-0897-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-0897-3