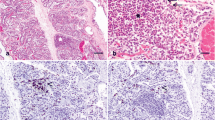
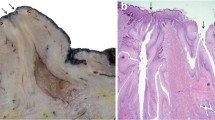
Abstract
It is a given in biology that structure and function go hand-in-hand. At the level of the mammary alveoli, copious milk production depends on the proliferation of mammary epithelial cells and the biochemical and structural differentiation of these cells after parturition. For example, data from quantitative structural studies demonstrate that differences in milk production between beef and dairy cows correspond with a relative failure of alveolar cell differentiation in cattle not specifically selected for milk yield. It is likely, but not proven, that production differences within or between dairy breeds are also determined by differences in the capacity of alveolar cells to differentiate or to maintain an adequate state of differentiation. These observations strongly support the belief that insults from mastitis that lead to losses in mammary function are directly related to disruption of alveolar cell integrity, sloughing of cells, induced apoptosis, and increased appearance of poorly-differentiated cells. Ironically, reduced milk production in cases of subclinical mastitis, is also associated with increases in milk somatic cell count. Thus the elevated neutrophil migration evoked to fight inflammation can inadvertently rendered alveolar epithelial cells non-secretory. A challenge to future researchers will be to devise mastitis treatments and therapies that prevent and/or repair damage to alveolar structure and maximize subsequent secretory cell differentiation.








Similar content being viewed by others
Abbreviations
- MSCC:
-
milk somatic cell count
- DHI:
-
Dairy Herd Improvement Association
- PMN:
-
polymorphonuclear leucocyte
- TNF-α:
-
tumor necrosis factor
References
Bramley AJ, Cullor JS, Erskine RJ, Fox LK, Harmon RJ, Hogan JS, et al. The mastitis problem. Current concepts of bovine mastitis. 4th ed. Madison: National Mastitis Council; 1996.
Jones GM, Pearson RE, Clabaugh GA, Heald CW. Relationships between somatic cell counts and milk production. J Dairy Sci. 1984;67:1823–31.
Akers RM, Thompson W. Effect of induced leucocyte migration on mammary cell morphology and milk component biosynthesis. J Dairy Sci. 1987;70:1685–95.
Capuco AV, Paape MJ, Nickerson SC. In vitro study of polymorphonuclear leukocyte damage to mammary tissue of lactating cows. Am J Vet Res. 1986;47:633–68.
Zhao X, Lacasse P. Mammary damage during mastitis: causes and control. J Anim Sci. 2008;86:57–68.
McFadden TB, Akers RM, Capuco AV. Relationships of milk proteins in blood with somatic cell counts in milk of dairy cows. J Dairy Sci. 1988;71:826–34.
McFadden TB, Akers RM, Kazmer GW. Alpha-lactalbumin in bovine serum: relationships with udder development and function. J Dairy Sci. 1987;70:259–64.
Akers RM. Lactation and the mammary gland. Iowa State Press Blackwell Publishing Company; 2002. 278 pages.
Akers RM. Major advances associated with hormones and growth factor regulation of mammary growth and lactation in dairy cows. J Dairy Sci. 2006;89:1222–34.
Tucker HA. Physiological control of mammary growth, lactogenesis, and lactation. J Dairy Sci. 1981;64:1403–21.
Tucker HA. Hormones, mammary growth, and lactation: a 41 year perspective. J Dairy Sci. 2000;70:1958–66.
Capuco AV, Akers RM. Lactation | galactopoiesis, effects of hormones and growth factors. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopedia of dairy sciences, vol. 2. 2nd ed. San Diego: Academic; 2011. p. 26–31.
Capuco, AV, Wood DL, Baldwin R, McLeod K, Paape MJ. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relationship to milk production and the effect of bST. J Dairy Sci. 2001;2177–87.
Capuco AV, Akers RM. The origin and evolution of lactation. J Biology. 2009;8:1–4.
Akers RM, Capuco AV, Keys JE. Cellular differentiation in mammary tissue of beef and dairy heifers. Livest Sci. 2006;105:44–9.
Keys JE, Capuco AV, Akers RM, Djiane J. Comparative study of mammary gland development and differentiation between beef and dairy heifers. Domest Anim Endocrinol. 1989;6:311–9.
Akers RM, Bauman DE, Goodman GT, Capuco AV, Tucker HA. Prolactin regulation of cytological differentiation of mammary epithelial cells in periparturient cows. Endocrinology. 1981;109:31–40.
Capuco AV, Akers RM, Smith JJ. Mammary growth in Holstein cows during the dry period: quantitation of nucleic acids and histology. J Dairy Sci. 1997;80:477–87.
Nickerson SC, Akers RM. Effect prepartum blockade of microtubule formation on ultrastructural differentiation of the mammary epithelium in Holstein heifers. Int J Biochem. 1983;15:777–88.
Filep R, Akers RM. Casein secretion and cytological differentiation in mammary tissue from bulls of high or low genetic merit. J Dairy Sci. 2000;83:2261–8.
Akers RM, Nickerson SC. Effect prepartum blockade of microtubule formation on milk production and biochemical differentiation of the mammary epithelium in Holstein heifers. Int J Biochem. 1983;15:771–5.
Helmbolt CF, Jungherr EL, Plaistridge WN. The histopathology of bovine mastitis. Storrs Agric Exp Stat Bull. 1953;505–95.
Spencer GR. The significance of hypersensitivity in bovine mastitis as determined by a study of its pathogenesis. Thesis. Univ. of Wisconsin. Madison, WI; 1949.
Pattison IH. The progressive pathology of bacterial mastitis. Vet Rec. 1958;70:114–7.
Chandler RL, Reid IM. Ultrastructural and associated observations in clinical cases of mastitis in cattle. J Comp Path. 1973;83:233–41.
Heald CW. Morphometric study of experimentally induced Staphylococcus bovis mastitis in the cow. Am J Vet Res. 1979;40:1294–8.
Capuco AV. Identification of putative bovine mammary epithelial stem cells by their retention of labeled DNA strands. Exp Biol Med. 2007;232:1381–90.
Nickerson SC. Histological response of the bovine mammary gland to experimental S. aureus infection. Ph.D. Thesis, Virginia Tech, Blacksburg, VA; 1980.
Nickerson SC, Heald CW. Histopathologic response of the bovine mammary gland to experimentally induced Staphylococcus aureus infection. Am J Vet Res. 1981;42:1351–5.
Harmon RJ, Heald CW. Migration of polymorphonuclear leukocytes into the bovine mammary gland during experimentally induced Staphylococcus aureus mastitis. Am J Vet Res. 1982;43:992–8.
Seelig LL, Beer AE. Transepithelial migration of leukocytes in the mammary gland of lactating rats. Biol Reprod. 1978;18:736–44.
Frost AJ, Hill AW, Brooker BE. The early pathogenesis of bovine mastitis due to Escherichia coli. Proc R Soc Lond B Biol Sci. 1980;209:431–9.
Nickerson SC, Heald CW. Cells in local reaction to experimental Staphylococcus aureus infection in bovine mammary gland. J Dairy Sci. 1982;65:105–16.
Nickerson SC, Pankey JW. Neutrophil migration through teat end tissues of bovine mammary quarters experimentally challenged with Staphylococcus aureus. J Dairy Sci. 1984;67:826–34.
Lin Y, Xia L, Turner JD, Zhao X. Morphologic observation of neutrophil diapedesis across bovine mammary gland epithelium in vitro. Am J Vet Res. 1995;56:203–7.
Kawanishi H. Morphologic association of lymphocytes with hepatocytes in chronic liver disease. Arch Pathol Lab Med. 1977;101:286–91.
McKenzie WN, Anderson RR. Endotoxin Induced migration of leukocytes from blood to milk. J Dairy Sci. 1981;64:227–35.
Nickerson SC, Pankey JW. Electron microscopic study of leucocytic infiltration of the mammary teat duct during infection with Staphylococcus aureus. Res Vet Sci. 1985;38:167–73.
Heald CW, Harmon RJ. Staphylotoxin induced cytolysis of mouse mammary alveoli at 2, 4, and 6 hours in vitro. JDS 1980; 63, Supplement 1, abstract no. 150
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akers, R.M., Nickerson, S.C. Mastitis and its Impact on Structure and Function in the Ruminant Mammary Gland. J Mammary Gland Biol Neoplasia 16, 275–289 (2011). https://doi.org/10.1007/s10911-011-9231-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-011-9231-3