Abstract
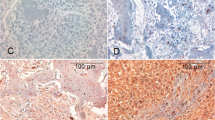
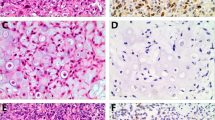
Canine primary bone tumors have a plastic radiographic image, demanding histopathological confirmation. Bone tumors are characterized by the type and amount of extracellular matrix produced what cannot be easily recognized, especially in biopsy samples. Identifying cellular markers that could aid diagnosis has supported various studies in oncological pathology. This study aimed to evaluate 22 canine primary bone neoplasms, establishing their histopathological diagnosis and evaluated vimentin, osteonectin and osteocalcin expression and their implication in diagnosis and prognosis. There were 12 productive osteoblastic osteosarcomas, six minimally productive osteoblastic osteosarcoma, two chondrosarcomas, one fibrosarcoma and one hemangiosarcoma. Immunostaining was cytoplasmatic in all cases, with average percentage of 87.9% for vimentin, 98.0% for osteonectin and 99.9% for osteocalcin. In this last case, only osteosarcomas were considered. Intensity was higher in vimentin labeling (+++), followed by osteonectin (++) and osteocalcin (+). One osteosarcoma showed negative immunostaining for vimentin and of samples submitted to anti-osteocalcin immunostaining, three osteosarcomas and one fibrosarcoma had negative staining. Besides identifying mesenchymal origin, vimentin elevated expression in canine bone tumors can be related to epithelial–mesenchymal transition, leading to more aggressive tumoral phenotypes and metastasis development. Similarly, high osteonectin expression is implicated in neoplastic cell invasion and is also related to metastasis spread. Decreased osteocalcin expression was found in some osteosarcoma samples and can be related to poor prognosis, as in human osteosarcomas. Our findings suggest that vimentin, osteonectin and osteocalcin not only aid diagnosis but can be related to prognosis in canine primary bone tumors, especially osteosarcomas and its osteoblastic subtype.


Similar content being viewed by others
References
Ehrhart NP, Ryan SD, Fan TM (2013) Tumors of the skeletal system. In: Withrow SJ, Vail DM, Page RL (eds) Withrow & MacEwen’s small animal clinical oncology. 5th edn. Elsevier Saunders Company, Missouri, pp 463–503
Sabattini S, Renzi A, Buracco P, Defourny S, Garnier-Moiroux M, Capitani O, Bettini G (2017) Comparative assessment of the accuracy of cytological and histologic biopsies in the diagnosis of canine bone lesions. J Vet Intern Med 31:864–871
Morello E, Martano M, Buracco P (2011) Biology, diagnosis and treatment of canine appendicular osteosarcoma: similarities and differences with human osteosarcoma. Vet J 189:268–277
Simpson S, Dunning MD, De Brot S, Grau-Roma L, Mongan NP, Rutland CS (2017) Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand 59:71
Fenger JM, London CA, Kisseberth WC (2014) Canine osteosarcoma: a naturally occurring disease to inform pediatric oncology. ILAR J 55:69–85
Fan TM, Khanna C (2015) Comparative aspects of osteosarcoma pathogenesis in humans and dogs. Vet Sci 2:210–230
Vanel M, Blond L, Vanel D (2013) Imaging of primary bone tumors in veterinary medicine: which differences? Eur J Radiol 82:2129–2139
Redondo A, Bagué S, Bernabeu D, Ortiz-Cruz E, Valverde C, Alvarez R, Martinez-Trufero J, Lopez-Martin JA, Correa R, Cruz J, Lopez-Pousa A, Santos A, Del Muro XG, Martin-Broto J (2017) Malignant bone tumors (other than Ewing’s): clinical practice guidelines for diagnosis, treatment and follow-up by Spanish Group for Research on Sarcomas (GEIS). Cancer Chemother Pharmacol 80:1113–1131
Niu X, Xu H, Inwards CY, Li Y, Ding Y, Letson GD, Bui MM (2015) Primary bone tumors: epidemiologic comparison of 9200 patients treated at Beijing Ji Shui Tan Hospital, Beijing, China, with 10.165 patients at Mayo Clinic, Rochester, Minnesota. Arch Pathol Lab Med 139:1149–1155
Slayter MV, Boosinger TR, Pool RR, Dämmrich K, Misdorp W, Larsen S (1994) Histological classification of bone and joint tumors of domestic animals. Armed Forces Institute of Pathology, Washington, DC
Fletcher CDM, Unni KK, Mertens F (2002) World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. International Agency for Research on Cancer, Lyon
Nagamine E, Hirayama K, Matsuda K, Okamoto M, Ohmachi T, Kadosawa T, Taniyama H (2015) Diversity of histologic patterns and expression of cytoskeletal proteins in canine skeletal osteosarcoma. Vet Pathol 52:977–984
Wehrle-Martinez AS, Dittmer KE, Aberdein D, Thompson KG (2016) Osteocalcin and osteonectin expression in canine osteosarcoma. Vet Pathol 53:781–787
Mesquita l, Mortier J, Ressel L, Finotello R, Silvestrini P, Piviani M (2017) Neoplastic pleural effusion and intrathoracic metastasis of a scapular osteosarcoma in a dog: a multidisciplinary integrated diagnostic approach. Vet Clin Pathol 46:337–343
Daugaard S, Christensen LH, Hogdall E (2009) Markers aiding the diagnosis of chondroid tumors: an immunohistochemical study including osteonectin, bcl-2, cox-2, actin, calponin, D2-40 (podoplanin), mdm-2, CD117 (c-kit) and YKL-40. APMIS 117:518–525
El-Badawi ZH, Muhammad EM, Noaman HH (2012) Role of immunohistochemical cyclo-oxygenase-2 (COX-2) and osteocalcin in differentiating between osteoblastomas and osteosarcomas. Malas J Pathol 34:15–23
Lamb S, Reavill D, Wojcieszyn J, Sitinas N (2014) Osteosarcoma of the tibiotarsus with possible pulmonary metastasis in a Ring-necked dove (Streptopelia risoria). J Avian Med Surg 28:50–56
Wijesundera KK, Izawa T, Fujita D, Denda Y, Seto E, Sasai H, Kuwamura M, Yamate J (2013) Spontaneous extraskeletal osteosarcoma in a rabbit (Oryctolagus cuniculus): histopathological and immunohistochemical findings. J Toxicol Pathol 26:309–312
Salyards GW, Blas-Machado U, Mishra S, Harvey SB, Butler AM (2013) Spontaneous osteoblastic osteosarcoma in a Mongolian gerbil (Meriones unguiculatus). Comp Med 63:62–66
Bord S (2003) Protein localization in wax-embedded and frozen sections of bone using immunhistochemistry. In: Helfrich MH, Ralston SH (eds) Bone research Protocols, 1st edn. Springer, New York, pp 237–247
Satelli A, Li S (2011) Vimentin as a potential molecular target in cancer therapy. Cell Mol Life Sci 68:3033–3046
Mendez MG, Kojima S, Goldman RD (2010) Vimentin induces changes in cell shape, motility and adhesion during the epithelial to mesenchymal transition. FASEB J 24:1838–1851
Schoumacher M, Goldman RD, Louvard D, Vignjevic DM (2010) Actin, microtubules and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 189:541–556
Li M, Zhang B, Sun B, Wang X, Ban X, Sun T, Liu Z, Zhao X (2010) A novel function for vimentin: the potential biomarker for predicting melanoma hematogenous metastasis. J Exp Clin Cancer Res 11:129
Shang Y, Li Z, Li H, Xia H, Lin Z (2013) TIM-3 expression in human osteosarcoma: correlation with the expression of epithelial–mesenchymal transition-specific biomarkers. Oncol Lett 6:490–494
Oblak ML, Boston SE, Woods JP, Nykamp S (2013) Comparison of concurrent imaging modalities for staging of dogs with appendicular primary bone tumours. Vet Comp Oncol 13:28–39
Eberle N, Fork M, Von Babo V, Nolte I, Simon D (2011) Comparison of examination of thoracic radiographs and thoracic computed tomography in dogs with appendicular osteosarcoma. Vet Comp Oncol 9:131–140
Yang Y, Niu X, Liu W, Xu H (2017) Expression and significance of secreted protein acidic and rich in cysteine in human osteosarcoma. Oncol Lett 14:5491–5496
Chlenski A, Cohn SL (2010) Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol 21:55–65
Ribeiro N, Sousa SR, Brekken RA, Monteiro FJ (2014) Role of SPARC in bone remodeling and cancer-related bone metastasis. J Cell Biochem 115:17–26
Chen H, Kolman K, Lanciloti N, Nerney M, Hays E, Robson C, Chandar N (2012) p53 and MDM2 are involved in the regulation of osteocalcin gene expression. Exp Cell Res 318:867–876
Kim YS, Paik IY, Rhie YJ, Suh SH (2010) Integrative physiology: defined novel metabolic roles of osteocalcin. J Korean Med Sci 25:985–991
Chen H, Hays E, Liboon J, Neely C, Kolman K, Chandar N (2011) Osteocalcin gene expression is regulated by wild-type p53. Calcif Tissue Int 89:411–418
Lee JS, Tung C (2013) Osteotropic cancer diagnosis by an osteocalcin inspired molecular imaging mimetic. Biochim Biophys Acta 1830:4621–4627
Won KY, Lee CH, Kim YW, Park YK (2011) Primary giant-cell-rich osteosarcoma of the urinary bladder: usefulness of osteocalcin and osteonectin immunohistochemical staining and literature review. Pathology 43:161–164
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The study was approved by the Universidade Federal Fluminense Ethics Committee in Animal Use under protocol nº 140 and all procedures were authorized by the owners after previous explanation. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amaral, C.B., Leite, J.d., Fonseca, A.B.M. et al. Vimentin, osteocalcin and osteonectin expression in canine primary bone tumors: diagnostic and prognostic implications. Mol Biol Rep 45, 1289–1296 (2018). https://doi.org/10.1007/s11033-018-4285-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4285-6