Abstract
Background
Our group recently reported that a mutation of the novel Beclin1 K414R acetylation site impacts the stability of Beclin1 protein, which decreases autophagy in adipocytes and further impedes adipocyte differentiation and lipolysis. This study was to explore whether Beclin1 acetylation plays a role in the early renal injury induced by high glucose and to further investigate the K414R mutation site in podocytes.
Methods
Male Sprague–Dawley rats were randomized to con (control) and diabetic nephropathy (DN) groups. The DN group was induced by a single 55 mg/kg intraperitoneal injection of streptozotocin and fed a high-fat and high-sugar diet (the con group received an equal volume of the vehicle and fed a plain diet), after 3 days of induction, blood glucose levels were measured to confirm the onset of diabetes. Then, at weeks 0 and 4, the biochemical index was assayed and renal cortex tissues were harvested. MPC5 podocytes were cultured in vitro. Beclin1 (K414R)-pLVX-ZsGreen1-N1(wild-type or mutant) lentiviral plasmids were transfected into podocytes. Western blot or immunoprecipitation was used to test proteins or the acetylation levels respectively, and immunohistochemistry was used to analyze morphological changes of podocytes. Immunofluorescence was used to detect the aggregation of LC3 puncta.
Results
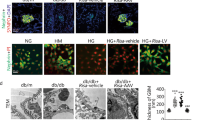
The acetylation level of Beclin1 was upregulated with podocyte injury exacerbated in high glucose at 24 h and that a mutation at K414R could inhibit hyperactivated autophagy, which ameliorated podocyte impairment.
Conclusion
These findings suggest that the acetylation site at K414 is a critical molecule and drug target and that further research into this area is warranted.




Similar content being viewed by others
Data availability
Data are available from the corresponding author on reasonable request.
Material availability
Data are available from the corresponding author on reasonable request.
References
Torban E, Braun F, Wanner N, Takano T, Goodyer PR, Lennon R, Ronco P, Cybulsky AV, Huber TB (2019) From podocyte biology to novel cures for glomerular disease. Kidney Int 96:850–861. https://doi.org/10.1016/j.kint.2019.05.015
Kobayashi R, Kamiie J, Yasuno K, Ogihara K, Shirota K (2011) Expression of nephrin, podocin, α-actinin-4 and α3-integrin in canine renal glomeruli. J Comp Pathol 145:220–225. https://doi.org/10.1016/j.jcpa.2011.01.007
Lin Q, Banu K, Ni Z, Leventhal JS, Menon MC (2021) Podocyte autophagy in homeostasis and disease. J Clin Med. https://doi.org/10.3390/jcm10061184
Bork T, Liang W, Yamahara K, Lee P, Tian Z, Liu S, Schell C, Thedieck K, Hartleben B, Patel K, Tharaux PL, Lenoir O, Huber TB (2020) Podocytes maintain high basal levels of autophagy independent of mtor signaling. Autophagy 16:1932–1948. https://doi.org/10.1080/15548627.2019.1705007
Hartleben B, Gödel M, Meyer-Schwesinger C, Liu S, Ulrich T, Köbler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstädt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB (2010) Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 120:1084–1096. https://doi.org/10.1172/jci39492
Cao AL, Wang L, Chen X, Wang YM, Guo HJ, Chu S, Liu C, Zhang XM, Peng W (2016) Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest 96:610–622. https://doi.org/10.1038/labinvest.2016.44
Lenoir O, Jasiek M, Hénique C, Guyonnet L, Hartleben B, Bork T, Chipont A, Flosseau K, Bensaada I, Schmitt A, Massé JM, Souyri M, Huber TB, Tharaux PL (2015) Endothelial cell and podocyte autophagy synergistically protect from diabetes-induced glomerulosclerosis. Autophagy 11:1130–1145. https://doi.org/10.1080/15548627.2015.1049799
Chen X, Pan Z, Fang Z, Lin W, Wu S, Yang F, Li Y, Fu H, Gao H, Li S (2018) Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. J Neuroinflamm 15:310. https://doi.org/10.1186/s12974-018-1345-8
Esteves AR, Filipe F, Magalhães JD, Silva DF, Cardoso SM (2019) The role of beclin-1 acetylation on autophagic flux in Alzheimer’s disease. Mol Neurobiol 56:5654–5670. https://doi.org/10.1007/s12035-019-1483-8
Sun T, Li X, Zhang P, Chen WD, Zhang HL, Li DD, Deng R, Qian XJ, Jiao L, Ji J, Li YT, Wu RY, Yu Y, Feng GK, Zhu XF (2015) Acetylation of beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat Commun 6:7215. https://doi.org/10.1038/ncomms8215
Li C, Xu J, Yu Q, Wang P, Dong B, Shen L, Wang Q, Li S, Yang Y, Deng Y (2021) Mutation of the novel acetylation site at K414R of BECN1 is involved in adipocyte differentiation and lipolysis. J Cell Mol Med. https://doi.org/10.1111/jcmm.16692
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010) Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160:1577–1579. https://doi.org/10.1111/j.1476-5381.2010.00872.x
Wang L, Du Y, Lu M, Li T (2012) ASEB: a web server for KAT-specific acetylation site prediction. Nucleic Acids Res 40:W376–W379. https://doi.org/10.1093/nar/gks437
Li JJ, Kwak SJ, Jung DS, Kim JJ, Yoo TH, Ryu DR, Han SH, Choi HY, Lee JE, Moon SJ, Kim DK, Han DS, Kang SW (2007) Podocyte biology in diabetic nephropathy. Kidney Int. https://doi.org/10.1038/sj.ki.5002384
Xu J, Deng Y, Wang Y, Sun X, Chen S, Fu G (2020) SPAG5-AS1 inhibited autophagy and aggravated apoptosis of podocytes via SPAG5/AKT/mTOR pathway. Cell Prolif 53:e12738. https://doi.org/10.1111/cpr.12738
Wang Q, Li R, Xiao Z, Hou C (2020) Lycopene attenuates high glucose-mediated apoptosis in MPC5 podocytes by promoting autophagy via the PI3K/AKT signaling pathway. Exp Ther Med 20:2870–2878. https://doi.org/10.3892/etm.2020.8999
Fu LL, Cheng Y, Liu B (2013) Beclin-1: autophagic regulator and therapeutic target in cancer. Int J Biochem Cell Biol 45:921–924. https://doi.org/10.1016/j.biocel.2013.02.007
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459. https://doi.org/10.1016/j.bbamcr.2013.06.001
Wu S, He Y, Qiu X, Yang W, Liu W, Li X, Li Y, Shen HM, Wang R, Yue Z, Zhao Y (2018) Targeting the potent beclin 1-UVRAG coiled-coil interaction with designed peptides enhances autophagy and endolysosomal trafficking. Proc Natl Acad Sci U S A 115:E5669-e5678. https://doi.org/10.1073/pnas.1721173115
Huang W, Choi W, Hu W, Mi N, Guo Q, Ma M, Liu M, Tian Y, Lu P, Wang FL, Deng H, Liu L, Gao N, Yu L, Shi Y (2012) Crystal structure and biochemical analyses reveal beclin 1 as a novel membrane binding protein. Cell Res 22:473–489. https://doi.org/10.1038/cr.2012.24
Dikic I, Elazar Z (2018) Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol 19:349–364. https://doi.org/10.1038/s41580-018-0003-4
Liu T, Tang Q, Liu K, Xie W, Liu X, Wang H, Wang RF, Cui J (2016) TRIM11 suppresses AIM2 inflammasome by degrading AIM2 via p62-dependent selective autophagy. Cell Rep 16:1988–2002. https://doi.org/10.1016/j.celrep.2016.07.019
Saitoh Y, Fujikake N, Okamoto Y, Popiel HA, Hatanaka Y, Ueyama M, Suzuki M, Gaumer S, Murata M, Wada K, Nagai Y (2015) p62 plays a protective role in the autophagic degradation of polyglutamine protein oligomers in polyglutamine disease model flies. J Biol Chem 290:1442–1453. https://doi.org/10.1074/jbc.M114.590281
Livingston MJ, Ding HF, Huang S, Hill JA, Yin XM, Dong Z (2016) Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 12:976–998. https://doi.org/10.1080/15548627.2016.1166317
Hazari Y, Bravo-San Pedro JM, Hetz C, Galluzzi L, Kroemer G (2020) Autophagy in hepatic adaptation to stress. J Hepatol 72:183–196. https://doi.org/10.1016/j.jhep.2019.08.026
Guo H, Wang B, Li H, Ling L, Niu J, Gu Y (2018) Glucagon-like peptide-1 analog prevents obesity-related glomerulopathy by inhibiting excessive autophagy in podocytes. Am J Physiol Renal Physiol 314:F181-f189. https://doi.org/10.1152/ajprenal.00302.2017
Wang W, Cai J, Tang S, Zhang Y, Gao X, Xie L, Mou Z, Wu Y, Wang L, Zhang J (2016) Sinomenine attenuates angiotensin II-induced autophagy via inhibition of P47-phox translocation to the membrane and influences reactive oxygen species generation in podocytes. Kidney Blood Press Res 41:158–167. https://doi.org/10.1159/000443417
He C, Liu G, Zhuang S, Zhang J, Chen Y, Li H, Huang Z, Zheng Y (2020) Yu Nu compound regulates autophagy and apoptosis through mTOR in vivo and vitro. Diabetes Metab Syndr Obes 13:2081–2092. https://doi.org/10.2147/dmso.S253494
Kang YL, Saleem MA, Chan KW, Yung BY, Law HK (2014) The cytoprotective role of autophagy in puromycin aminonucleoside treated human podocytes. Biochem Biophys Res Commun 443:628–634. https://doi.org/10.1016/j.bbrc.2013.12.015
Su Y, Yao S, Zhao S, Li J, Li H (2020) LncRNA CCAT1 functions as apoptosis inhibitor in podocytes via autophagy inhibition. J Cell Biochem 121:621–631. https://doi.org/10.1002/jcb.29307
Lioudaki E, Stylianou KG, Petrakis I, Kokologiannakis G, Passam A, Mikhailidis DP, Daphnis EK, Ganotakis ES (2015) Increased urinary excretion of podocyte markers in normoalbuminuric patients with diabetes. Nephron 131:34–42. https://doi.org/10.1159/000438493
Regoli M, Bendayan M (1997) Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia 40:15–22. https://doi.org/10.1007/s001250050637
Chen HC, Chen CA, Guh JY, Chang JM, Shin SJ, Lai YH (2000) Altering expression of alpha3beta1 integrin on podocytes of human and rats with diabetes. Life Sci 67:2345–2353. https://doi.org/10.1016/s0024-3205(00)00815-8
Chen CA, Tsai JC, Su PW, Lai YH, Chen HC (2006) Signaling and regulatory mechanisms of integrinalpha3beta1 on the apoptosis of cultured rat podocytes. J Lab Clin Med 147:274–280. https://doi.org/10.1016/j.lab.2005.12.010
Acknowledgements
This study was supported by grants from the National Natural Science Fund Youth Project of China (No. 81600684 and No. 81603476), as well as the Youth Fund Project of the Shangdong Natural Science Foundation (ZR2016HQ16). We thank Professor Ying Yang from the Shanghai Key Laboratory of Diabetes for scientific advice.
Author information
Authors and Affiliations
Contributions
All authors took responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussion. BS designed the study. JX, YD, and PW analyzed the results, and YK, YZ and QY wrote and edited the manuscript. BS and CL reviewed and edited the manuscript. JX, YD and WP reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The experimental protocol was approved by the Ethics Committee for Animal Experimentation of The Affiliated Hospital of Qingdao University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, J., Deng, Y., Ke, Y. et al. Mutation of Beclin1 acetylation site at K414 alleviates high glucose-induced podocyte impairment in the early stage of diabetic nephropathy by inhibiting hyperactivated autophagy. Mol Biol Rep 49, 3919–3926 (2022). https://doi.org/10.1007/s11033-022-07242-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-022-07242-2