Abstract
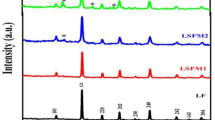
Iron-nickel alloys have been synthesized through thermal decomposition of nitrates aqueous solutions followed by H2 reduction. The effect of temperature [773 K to 973 K (500 °C to 700 °C)] over the oxide reduction kinetics has been studied. The results indicate that at 973 K (700 °C) and for a reaction time of 90 minutes, it was possible to obtain Fe-Ni alloys with distinct compositions. The X-ray diffraction analysis (XRD) of the initial oxide mixture has shown distinct peaks of iron and nickel oxides (Fe2O3, NiO, and Fe2NiO4). The same analysis for the reduced samples suggests that alloying has taken place. Indeed, the Rietveld analysis of the XRD patterns indicates the presence of two solid solutions—alpha (BCC) and FeNi3 (FCC), with varying mass percent depending on the initial Fe to Ni ratio, in accordance with the information contained in the Fe-Ni phase diagram. The calculated alloy average crystallite size has shown to be equal to 26 nm. Total magnetization measurements suggest a superparamagnetic behavior, which is a typical result for magnetic materials of considerable nanostructured content, in accordance with the expectation based on the quantitative evaluation of the XRD data.











Similar content being viewed by others
References
G. Herzer, IEEE Trans. Magn. 25 (1989), 3327−3329.
C. P. Bean, J. D. Livingston, Journal of Applied Physics 30 4 (1959) 120−129.
R. P. Cowburn, Journal of Applied Physics 93 11 (2003) 9310−9315.
T. Yokoyama, K. Eguchi, Phys. Rev. Lett. 2011, 107 (6), pp. 065901-1–065901-4.
N. Ballot, F. Schoenstein, S. Mercone, T. Chauveau, O. Brinza, N. Jouini, Journal of Alloys and Compounds Volume 536 (2012) 381–385.
M.H. Xu, W. Zhong, X.S. Qi, C.T. Au, Y. Deng, Y.W. Du, Journal of Alloys and Compounds 495 (2010) 200−204.
M. Bahgat, M.K. Paek, J.J. Pak, Journal of Alloys and Compounds 466 (2008) 59–66.
D.H. Riu, S.T. Oh, Current Applied Physics Volume 12 (2012) 188–191.
H.Q. Wu, Y.J. Cao, P.S. Yuan, H.Y. Xu, X.W. Wei, Chem. Phys. Lett. 406 (2005) 148–53.
S.W. Du, R.V. Ramanujan, Journal of Magnetism and Magnetic Materials 292 (2005) 286−298.
L.H. Zhu, X.M. Ma, L. Zhao, Journal of Materials Science 36 23 (2001) 5571−5574.
Y. Liu, J. Zhang, L. Yu, G. Jia, C. Jing, S. Cao, J. Magn. Magn. Mater. 285 (2005) 138–44.
E.A. Brocchi, M.S. Motta, P.K. Jena, (2004) Metall. Mater. Trans. B, 35B, pp. 1107–12.
P.K. Jena, E.A. Brocchi, M.S. Motta, Materials Science & Engineering A 313 (2001) 180−186.
E.A. Brocchi, M.S. Motta, I.G. Solórzano, P.K. Jena, F.J. Moura Materials Science and Engineering B Volume 112 Issue 2-3 (2004) 200−205.
G. Cacciamani, J. De Keyzer, R. Ferro, U.E. Klotz, J. Lacaze, P. Wollants, Intermetallics, 14 (2006) 1312−1325.
E. A. Brocchi, R. C. S. Navarro, M.S. Motta, F. J. Moura, I.G. Solórzano. Materials Chemistry and Physics, 140 (2013) 273 – 283.
O.A. Cortez: Doctorate Thesis, Materials Engineering Department, Catholic University of Rio de Janeiro, Rio de Janeiro, Brazil, 2008.
A. Lutts, P.M. Gielen, Physica Status Solidi 41 (1970) 81−84.
Acknowledgments
The authors are grateful to CNPq, FAPERJ, and CAPES for the financial support and scholarships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted February 4, 2014.
Rights and permissions
About this article
Cite this article
Cortez, O.A., Moura, F.J., de Albuquerque Brocchi, E. et al. Fe-Ni Alloy Synthesis Based on Nitrates Thermal Decomposition Followed by H2 Reduction. Metall Mater Trans B 45, 2033–2039 (2014). https://doi.org/10.1007/s11663-014-0221-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-014-0221-x