Abstract
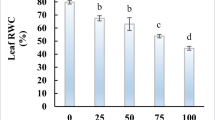
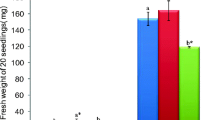
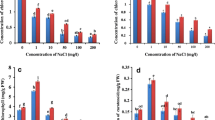
The physiological and antioxidant response to salinity was studied in pomegranate (Punica granatum L.) by exposing in vitro growing shoots of the Italian variety Profeta Partanna to 125 or 250 mM NaCl for 10 and 20 days. 250 mM NaCl significantly reduced shoot length, leaf area and water content of the shoots, regardless the length of the salt treatment,with respect to the control and to the 125 mM NaCl treatment. After 20 days the shoots treated with 250 mM NaCl also showed a significant reduction in relative growth rate (RGR) together with marked necroses and abscission of the oldest leaves. Salt treatments significantly decreased the contents of chlorophylls and carotenoids in both exposure times, depending on NaCl concentration. Proline, total phenolic compounds and ellagic acid did not increase or even decrease with the salt treatments. The levels of lipid peroxidation decreased, ascorbate peroxidase (APX) activity significantly increased in both treatment times and concentrations, while guaiacol peroxidase (G-POD) activity significantly increased in shoots treated with 250 mM NaCl for 20 days suggesting the rapid involvement of APX in controlling the oxidative stress in this species, even at low salt concentrations, and a delayed complementary role of G-POD.








Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- d.w.:
-
Dry weight
- f.w.:
-
Fresh weight
- G-POD:
-
Guaiacol peroxidase
- MDA:
-
Malondialdehyde
- MKI:
-
McKinney index
- RGR:
-
Relative growth rate
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
References
Adhami VM, Khan N, Mukhtar H (2009) Cancer chemoprevention by pomegranate: laboratory and clinical. Evid Nutr Cancer 61:811–815
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Azevedo Neto AD, Prico JT, Eneas-Filho J, Braga de Abreu CE, Gomes-Filho E (2006) Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ Exp Bot 56:235–241
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–28
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Booker FL, Miller JE (1998) Phenylpropanoid metabolism and phenolic composition of soybean [Glycine max (L.) Merr.] leaves following exposure to ozone. J Exp Bot 49:1191–1202
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brunetti C, Di Ferdinando M, Fini A, Pollastri S, Tattini M (2013) Flavonoids as antioxidants and developmental regulators: relative significance in plants and humans. Int J Mol Sci 14:3540–3555
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017) Reactive oxygen species, abiotic stress and stress combination. Plant J 90:856–867
Cram WJ (1976) Negative feedback regulation of transport in cells. The maintenance of turgor, volume and nutrient supply. Encycl Plant Physiol 2:284–316
Cuartero J, Bolarín MC, Asíns MJ, Moreno V (2006) Increasing salt tolerance in the tomato. J Exp Bot 57:1045–1058
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91
Di Cori P, Lucioli S, Frattarelli A, Nota P, Tel-Or E, Benyamini E, Gottlieb H, Caboni E, Forni C (2013) Characterization of the response of in vitro cultured Myrtus communis L. plants to high concentrations of NaCl. Plant Physiol Biochem 73:420–426
El-Agamy SZ, Mostafa RAA, Shaaban MM, El-Mahdy MT (2010) In vitro salt and drought tolerance of Manfalouty and Nab El-Gamal pomegranate cultivars. Aust J Basic Appl Sci 4:1076–1082
Erturk U, Sivritepe N, Yerlikaya C, Bor M, Ozdemir F, Turkan I (2007) Response of the cherry rootstock to salinity in vitro. Biol Plant 51:597–600
FAO (2015) Status of the world’s soil resources. http://www.fao.org/3/a-i5199e.pdf. Accessed 3 Jan 2017
Ferrara G, Giancaspro A, Mazzeo A, Giove SL, Matarrese AMS, Pacucci C, Punzi R, Trani A, Gambacorta G, Blanco A, Gadaleta A (2014) Characterization of pomegranate (Punica granatum L.) genotypes collected in Puglia region, Southeastern Italy. Sci Hortic 178:70–78
Forni C, Duca D, Glick BR (2017) Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 410:335–356
Gentile A, Frattarelli A, Nota P, Condello E, Caboni E (2016) The aromatic cytokinin meta-topolin promotes in vitro propagation, shoot quality and micrografting in Corylus colurna L. Plant Cell Tiss Organ Cult 128:693–703
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Golldack D, Li C, Mohan H, Probst N (2014) Tolerance to drought and salt stress in plants: unraveling the signaling networks. Front Plant Sci 5:151. https://doi.org/10.3389/fpls.2014.00151
Gumienna M, Szwengiel A, Górna B (2016) Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur Food Res Technol 242:631–640
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genom 2014:701596. https://doi.org/10.1155/2014/701596
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance. Mechanisms and their potential use for breeding. Front Plant Sci 7:1787. https://doi.org/10.3389/fpls.2016.01787
Hasanpour Z, Karimi HR, Mirdehghan SH (2015) Effects of salinity and water stress on echophysiological parameters and micronutrients concentration of pomegranate (Punica granatum L.). J Plant Nutr 38:1–13
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments: a review. Plant Signal Behav 7:1456–1466
Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H (2001) A large family of class III plant peroxidases. Plant Cell Physiol 42:462–468
Hodges DM, De Long JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42
Hossain MS, Dietz KJ (2016) Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front Plant Sci 7:548. https://doi.org/10.3389/fpls.2016.00548
Huang Z, Zhao L, Chen D, Liang M, Liu Z, Shao H, Long X (2013) Salt Stress Encourages proline accumulation by regulating proline biosynthesis and degradation in Jerusalem artichoke plantlets. PLoS One 8(4):e62085. https://doi.org/10.1371/journal.pone.0062085
Hummer KE, Pomper K, Postman JD, Graham CJ, Stover EW, Mercure EW, Aradhya MK, Crisosto CH, Ferguson L, Thompson M, Byers P, Zee FT (2012) Emerging fruit crops. In: Badenes ML, Byrne DH (eds) fruit breeding, handbook of plant breeding vol 8. Springer, Berlin, pp 97–147
Jaspers P, Kangasjärvi J (2010) Reactive oxygen species in abiotic stress signaling. Physiol Plant 138:405–413
Jbir-Koubaa R, Charfeddine S, Ellouz W, Saidi MN, Gargouri-Bouzid R, Nouri-Ellouz O (2015) Investigation of the response to salinity and to oxidative stress of interspecific potato somatic hybrids grown in a greenhouse. Plant Cell Tiss Organ Cult 120:933–947
Khan MH, Panda SK (2008) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89
Khayyat M, Tehranifar A, Davarynejad GH, Sayyari-Zahan MH (2014) Vegetative growth, compatible solute accumulation, ion partitioning and chlorophyll fluorescence of ‘Malas-e-Saveh’ and ‘Shishe-Kab’ pomegranates in response to salinity stress. Photosynthetica 52:301–312
Legrand B (1977) Action de la lumière sur les peroxydases et sur les teneursen composes phénoliques de tissus de feuilles de Cichorium intybus L. cultivés in vitro. Biol Plant 19:27–33
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lutts S, Kinet JM, Bouharmont J (1996) Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice cultivars differing in salt resistance. Plant Growth Regul 19:207–218
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Mastrogiannidou E, Chatzissavvidis C, Antonopoulou C, Tsabardoukas V, Giannakoula A, Therios I (2016) Response of pomegranate cv. Wonderful plants tο salinity. Soil Sci Plant Nut 6:621–636
McKinney HH (1923) Influence of soil temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J Agri Res 26:195–217
Mehlhorn H, Lelandais M, Korth HG, Foyer CH (1996) Ascorbate is the natural substrate for plant peroxidases. FEBS Lett 378:203–206
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ 33:453–467
Mishra P, Bhoomika K, Dubey RS (2013) Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 250:3–19
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55:1105–1113
Møller IM, Jensen PE, Hansson A (2007) Oxidative modifications to cellular components in plants. Annu Rev Plant Biol 58:459–481
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Niknam V, Meratan AA, Ghaffari SM (2011) The effect of salt stress on lipid peroxidation and antioxidative enzymes in callus of two Acanthophyllum species. In Vitro Cell Dev Biol-Plant 47:297–308
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid Metabolism in Plants. Mol Plant 8:68–82
Özer Ö, Mutlu B, Kıvçak B (2007) Antityrosinase activity of some plant extracts and formulations containing ellagic acid. Pharm Biol 45:519–524
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60:324–349
Piwowarczyk B, Tokarz K, Kamińska I (2016) Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell Tiss Organ Cult 124:227–240
Poustini K, Siosemardeh A, Ranjbar M (2007) Proline accumulation as a response to salt stress in 30 wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Genet Resour Crop Evol 54:925–934
Rossi L, Borghi M, Francini A, Linb X, Xie DY, Sebastiani L (2016) Salt stress induces differential regulation of the phenylpropanoid pathway in Olea europaea cultivars Frantoio (salt-tolerant) and Leccino (salt-sensitive). J Plant Physiol 204:8–15
Sepulveda E, Galleti L, Saenz C, Tapia M (2000) Minimal processing of pomegranate var. Wonderful Options Méditerr Ser A 42:237–242
Shalata A, Mittova V, Volokita M, Guy M, Tal M (2001) Response of cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: the root antioxidative system. Physiol Plantarum 122:487–494
Sharma P, Jah AB, Dubey RS, Pessarki M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Sheikh-Mohamadi MH, Etemadi N, Nikbakht A, Arab M, Majidi MM, Pessarakli M (2017) Antioxidant defence system and physiological responses of Iranian crested wheatgrass (Agropyron cristatum L.) to drought and salinity stress. Acta Physiol Plant 39:245. https://doi.org/10.1007/s11738-017-2543
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Syed DN, Afaq F, Mukhtar H (2007) Pomegranate derived products for cancer chemoprevention. Semin Cancer Biol 17:377–385
Tattini M, Remorini D, Pinelli P, Agati G, Saracini E, Traversi ML, Massai R (2006) Morpho-anatomical, physiological and biochemical adjustments in response to root zone salinity stress and high solar radiation in two Mediterranean evergreen shrubs, Myrtus communis and Pistacia lentiscus. N Phytol 170:779–794
Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK (2011) Additive effects of Na+ and Cl– ions on barley growth under salinity stress. J Exp Bot 62:21892203
Teixeira da Silva JA, Rana TS, Narzary D, Verma N, Meshram DM, Ranade SA (2013) Pomegranate biology and biotechnology: a review. Sci Hortic 160:85–107
Tuteja N (2007) Mechanisms of high salinity tolerance in plants. Methods Enzymol 428:419–438
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759
Wang H, Tang X, Wang H, Shao HB (2015) Proline accumulation and metabolism-related genes expression profiles in Kosteletzkya virginica seedlings under salt stress. Front Plant Sci 6:792. https://doi.org/10.3389/fpls.2015.00792
Watanabe S, Kojima K, Ide Y, Sasaki S (2000) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tiss Organ Cult 63:199–206
Woodward AJ, Bennett IJ (2005) The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell Tiss Organ Cult 82:189–200
You J, Chan Z (2015) ROS regulation during abiotic stress responses in crop plants. Front Plant Sci 6:1092. https://doi.org/10.3389/fpls.2015.01092
Zarfeshany A, Asgary S, Javanmard SH (2014) Potent health effects of pomegranate. Adv Biomed Res 3:100. https://doi.org/10.4103/2277-9175.129371
Zeng F, Shabala L, Zhou M, Zhang G, Shabala S (2013) Barley responses to combined waterlogging and salinity stress: separating effects of oxygen deprivation and elemental toxicity. Front Plant Sci 4:313. https://doi.org/10.3389/fpls.2013.00313
Acknowledgements
This work was partially supported by the Italian Agricultural Ministry ("RGV-FAO" Project).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Bartosz.
Rights and permissions
About this article
Cite this article
Urbinati, G., Nota, P., Frattarelli, A. et al. Morpho-physiological and antioxidant response to NaCl-induced stress in in vitro shoots of pomegranate (Punica granatum L.). Acta Physiol Plant 40, 151 (2018). https://doi.org/10.1007/s11738-018-2726-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2726-4