Abstract
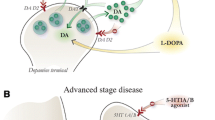
Although they are effective at treating the motor impairments that are the core symptoms of Parkinson’s disease, current treatments, namely l-3,4-dihydroxyphenylalanine (l-DOPA), the gold standard medication and high-frequency stimulation of the subthalamic nucleus (HFS-STN), can lead to cognitive and mood alterations. Many of these side effects, such as depression, anxiety and sleep disturbances, could be related to abnormal functioning of the serotonergic system, but much basic research remains to be done. Molecular studies in humans and animal models of the disease have reported diverse drastic changes to the serotonergic system. It has also been shown that the serotonergic system both plays a major role in the mechanism of action of the current therapies and is altered by the therapies. It has been reported that HFS-STN decreases serotonin release in several regions, mostly via inhibition of serotonergic neuron activity. The involvement of serotonergic neurons in l-DOPA treatment is even more significant. First, serotonergic neurons, able to convert exogenous l-DOPA to dopamine, are a major site to release dopamine throughout the brain. Second, the substitution of serotonin by newly synthesized dopamine in serotonin neurons leads to acute and chronic alteration of serotonin release and metabolism. Therefore, both therapeutic approaches, via distinct mechanisms, decrease serotonergic system activity and, rather than alleviating cognitive or mood disorders, tend to aggravate them. Molecular strategies targeting the serotonergic system are being developed and could be decisive in limiting l-DOPA-induced dyskinesia, as well as mood and cognitive symptoms produced by antiparkinsonian therapies.




Similar content being viewed by others
References
Melamed E, Zoldan J, Friedberg G, Ziv I, Weizmann A (1996) Involvement of serotonin in clinical features of Parkinson’s disease and complications of L-DOPA therapy. Adv Neurol 69:545–550
McDonald WM, Richard IH, DeLong MR (2003) Prevalence, etiology, and treatment of depression in Parkinson’s disease. Biol Psychiatry 54:363–375
Tandberg E, Larsen JP, Aarsland D, Cummings JL (1996) The occurrence of depression in Parkinson’s disease. A community-based study. Arch Neurol 53:175–179
Schuurman AG, van den Akker M, Ensinck KT, Metsemakers JF, Knottnerus JA, Leentjens AF, Buntinx F (2002) Increased risk of Parkinson’s disease after depression: a retrospective cohort study. Neurology 58:1501–1504
Shiba M, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA (2000) Anxiety disorders and depressive disorders preceding Parkinson’s disease: a case–control study. Mov Disord 15:669–677
Eskow Jaunajars KL, Angoa-Perez M, Kuhn DM, Bishop C (2011) Potential mechanisms underlying anxiety and depression in Parkinson’s disease: consequences of L-DOPA treatment. Neurosci Biobehav Rev 35:556–564
Steinbusch HW (1984) Serotonin-immunoreactive neurons and their projections in the CNS. In: Björklund A, Hökfelt T, Kuhar MJ (eds) Handbook of chemical neuroanatomy—classical transmitters and transmitters receptors in the CNS Part II. Elsevier, Amsterdam, pp 68–125
Kish SJ (2003) Biochemistry of Parkinson’s disease: is a brain serotonergic deficiency a characteristic of idiopathic Parkinson’s disease? Adv Neurol 91:39–49
Scholtissen B, Verhey FR, Steinbusch HW, Leentjens AF (2006) Serotonergic mechanisms in Parkinson’s disease: opposing results from preclinical and clinical data. J Neural Transm 113:59–73
Birkmayer W, Birkmayer JD (1987) Dopamine action and disorders of neurotransmitter balance. Gerontology 33:168–171
Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y (2008) Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 131:120–131
Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y (1983) Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res 275:321–328
Kerenyi L, Ricaurte GA, Schretlen DJ, McCann U, Varga J, Mathews WB, Ravert HT, Dannals RF, Hilton J, Wong DF, Szabo Z (2003) Positron emission tomography of striatal serotonin transporters in Parkinson disease. Arch Neurol 60:1123–1129
Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, Piccini P (2010) Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis 40:216–221
Caretti V, Stoffers D, Winogrodzka A et al (2008) (2008) Loss of thalamic serotonin transporters in early drug-naïve Parkinson’s disease patients is associated with tremor: an [(123)I]beta-CIT SPECT study. J Neural Transm 115:721–729
Roselli F, Pisciotta NM, Pennelli M, Aniello MS, Gigante A, De Caro MF, Ferrannini E, Tartaglione B, Niccoli-Asabella A, Defazio G, Livrea P, Rubini G (2010) Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov Disord 25:1853–1859
Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ (2003) Tremor in Parkinson’s disease and serotonergic dysfunction: an 11C-WAY 100635 PET study. Neurology 60:601–605
Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB (1990) Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107
Jellinger KA (1991) Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14:153–197
Kovacs GG, Klöppel S, Fischer I, Dorner S, Lindeck-Pozza E, Birner P, Bötefür IC, Pilz P, Volk B, Budka H (2003) Nucleus-specific alteration of raphe neurons in human neurodegenerative disorders. Neuroreport 14:73–76
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behaviour in rats after 6-hydroxy-dopamine lesion of the nigrostriatal dopamine system. Brain Res 24:485–493
Henry B, Crossman AR, Brotchie JM (1998) Characterization of enhanced behavioural responses to L-DOPA following repeated administration in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Exp Neurol 151:334–342
Ungerstedt U (1971) Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand Suppl 367:49–68
Zetterstrom T, Herrera-Marschitz M, Ungerstedt U (1986) Simultaneous measurement of dopamine release and rotational behaviour in the 6-hydroxydopamine denervated rats using intracerebral dialysis. Brain Res 376:1–7
Herrera-Marschitz M, Arbuthnott G, Ungerstedt U (2010) The rotational model and microdialysis: significance for dopamine signalling, clinical studies and beyond. Prog Neurobiol 90:176–189
Pearce RK, Jackson M, Smith L, Jenner P, Marsden CD (1995) Chronic L-DOPA administration induces dyskinesias in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmoset (Callithrix Jacchus). Mov Disord 10:731–740
Cenci MA, Ohlin KE (2009) Rodent models of treatment-induced motor complications in Parkinson’s disease. Parkinsonism Relat Disord 15:S13–S17
Olanow CW, Obeso JA (2000) Preventing levodopa-induced dyskinesias. Ann Neurol 47(Suppl 1):S167–S176
de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127:2747–2754
Cenci MA, Lundblad M (2006) Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J Neurochem 99:381–392
Cenci MA (2007) L-DOPA-induced dyskinesia: cellular mechanisms and approaches to treatment. Parkinsonism Relat Disord 13:S263–S267
Perez-Otano I, Herrero MT, Oset C, De Ceballos ML, Luguin MR, Obseo JA, Del Rio J (1991) Extensive loss of dopamine and serotonin induced by chronic administration of MPTP in the marmoset. Brain Res 567:127–132
Pifl C, Schingnitz G, Hornykiewicz O (1991) Effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 44:591–605
Russ H, Mihatsch W, Gerlach M, Riederer P, Przuntek H (1991) Neurochemical and behavioural features induced by chronic low dose treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the common marmoset: implications for Parkinson’s disease? Neurosci Lett 123:115–118
Boulet S, Mounayar S, Poupard A et al (2008) Behavioral recovery in MPTP-treated monkeys: neurochemical mechanisms studied by intrastriatal microdialysis. J Neurosci 28:9575–9584
Hara K, Tohyama I, Kimura H, Fukuda H, Nakamura S, Kameyama M (1987) Reversible serotoninergic neurotoxicity of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in mouse striatum studied by neurochemical and immunohistochemical approaches. Brain Res 410:371–374
Nayyar T, Bubser M, Ferguson MC, Neely MD, Shawn Goodwin J, Montine TJ, Deutch AY, Ansah TA (2009) Cortical serotonin and norepinephrine denervation in parkinsonism: preferential loss of beaded serotonin innervation. Eur J Neurosci 30:207–216
Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, Launay JM, Cohen-Salmon C, Hirsch EC (2003) Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol Dis 14:218–228
Vuckovic MG, Wood RI, Holschneider DP, Abernathy A, Togasaki DM, Smith A, Petzinger GM, Jakowec MW (2008) Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis 32:319–327
Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL (1998) Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci Lett 245:151–154
Breese GR, Baumeister AA, McCown TJ, Emerick SG, Frye GD, Crotty K, Muller RA (1984) Behavioral differences between neonatal and adult 6-hydroxydopamine-treated rats to dopamine agonists: relevance to neurological symptoms in clinical syndromes with reduced brain dopamine. J Pharmacol Exp Ther 231:343–354
Erinoff L, Snodgrass SR (1986) Effects of adult or neonatal treatment with 6-hydroxydopamine or 5,7-dihydroxytryptamine on locomotor activity, monoamine levels, and response to caffeine. Pharmacol Biochem Behav 24:1039–1045
Iwamoto ET, Loh HH, Way EL (1976) Circling behavior in rats with 6-hydroxydopamine or electrolytic nigral lesions. Eur J Pharmacol 37:339–356
Zhou FC, Bledsoe S, Murphy J (1991) Serotonergic sprouting is induced by dopamine-lesion in substantia nigra of adult rat brain. Brain Res 556:108–116
Commins DL, Shaughnessy RA, Axt KJ, Vosmer G, Seiden LS (1989) Variability among brain regions in the specificity of 6-hydroxydopamine (6-OHDA)-induced lesions. J Neural Transm 77:197–210
Kaya AH, Vlamings R, Tan S, Lim LW, Magill PJ, Steinbusch HW, Visser-vanderwalle V, Sharp T, Temel Y (2008) Increased electrical and metabolic activity in the dorsal raphe nucleus of parkinsonian rats. Brain Res 1221:93–97
Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HW, Visser-Vandewalle V, Sharp T (2007) Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci U S A 104:17087–17092
Wang S, Zhang GJ, Liu J, Wu ZH, Wang T, Gui ZH, Chen L, Wang Y (2009) Unilateral lesion of the nigrostriatal pathway induces an increase of neuronal firing of the midbrain raphe nuclei 5-HT neurons and a decrease of their response to 5-HT(1A) receptor stimulation in the rat. Neuroscience 159:850–861
Wang S, Zhang GJ, Liu J, Wu ZH, Ali U, Wang Y, Chen L, Gui ZH (2009) The firing of pyramidal neurons in medial prefrontal cortex and their response to 5-hydroxytryptamine-1A receptor stimulation in a rat model of Parkinson’s disease. Neuroscience 162:1091–1100
Wang S, Zhang QJ, Liu J, Ali U, Wu ZH, Chen L, Gui ZH, Wang Y, Hui YP (2009) In vivo effects of activation and blockade of 5-HT(2A/2C) receptors in the firing activity of pyramidal neurons of medial prefrontal cortex in a rodent model of Parkinson’s disease. Exp Neurol 219:239–248
Takeuchi Y, Sawada T, Blunt S, Jenner P, Marsden CD (1991) Serotonergic sprouting in the neostriatum after intrastriatal transplantation of fetal ventral mesencephalon. Brain Res 551:171–177
Guiard BP, El Mansari M, Merali Z, Blier P (2008) Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol 11:625–639
Bejjani BP, Damier P, Arnulf I et al (1999) Transient acute depression induced by high-frequency deep-brain stimulation. N Engl J Med 340:1476–1480
Deuschl G, Fogel W, Hahne M et al (2002) Deep-brain stimulation for Parkinson’s disease. J Neurol 249(suppl 3):36–39
Houeto JL, Damier P, Bejjani PB et al (2000) Subthalamic stimulation in Parkinson disease. Arch Neurol 57:461–465
Tan SK, Hartung H, Visser-Vandewalle V, Steinbusch HW, Temel Y, Sharp T (2011) A combined in vivo neurochemical and electrophysiological analysis of the effect of high-frequency stimulation of the subthalamic nucleus on 5-HT transmission. Exp Neurol. doi:10.1016/j.expneurol.2011.08.027
Thobois S, Mertens P, Guenot M et al (2002) Subthalamic nucleus stimulation in Parkinson’s disease. J Neurol 249:529–534
Kulisevsky J, Berthier ML, Gironell A et al (2002) Mania following deep brain stimulation for Parkinson’s disease. Neurology 59:1421–1424
Doshi PK, Chaya N, Bhatt MH (2002) Depression leading to attempted suicide after subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord 17:1084–1100
Houeto JL, Mesnage V, Mallet L et al (2002) Behavioral disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry 72:701–707
Saint-Cyr JA, Trépanier LL, Kumar R et al (2000) Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain 123:2091–2108
Navailles S, Benazzouz A, Bioulac B, Gross C, De Deurwaerdère P (2010) High-frequency stimulation of the subthalamic nucleus and L-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson’s disease. J Neurosci 30:2356–2364
Tan SK, Janssen ML, Jahanshahi A, Chouliaras L, Visser-Vandewalle V, Lim LW, Steinbusch HW, Sharp T, Temel Y (2011) High frequency stimulation of the subthalamic nucleus increases c-fos immunoreactivity in the dorsal raphe nucleus and afferent brain regions. J Psychiatr Res 45:1307–1315
Amilhon B, Lepicard E, Renoir T, Mongeau R, Popa D, Poirel O, Miot S, Gras C, Gardier AM, Gallego J, Hamon M, Lanfumey L, Gasnier B, Giros B, El Mestikawy S (2010) VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J Neurosci 30:2198–2210
Hale MW, Lowry CA (2011) Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 213:243–264
Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P (2011) A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J Neurosci 31:2756–2768
McQuade R, Sharp T (1997) Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem 69:791–796
Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K (2009) Optical deconstruction of parkinsonian neural circuitry. Science 324:354–359
Sesia T, Bulthuis V, Tan S, Lim LW, Vlamings R, Blokland A, Steinbusch HW, Sharp T, Visser-Vandewalle V, Temel Y (2010) Deep brain stimulation of the nucleus accumbens shell increases impulsive behavior and tissue levels of dopamine and serotonin. Exp Neurol 225:302–309
Hartung H, Tan SK, Steinbusch HM, Temel Y, Sharp T (2011) High-frequency stimulation of the subthalamic nucleus inhibits the firing of juxtacellular labelled 5-HT-containing neurones. Neuroscience 186:135–145
Cotzias GC (1968) L-Dopa for Parkinsonism. N Engl J Med 278:630
Hornykiewicz O (1966) Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev 18:925–964
Miller DW, Abercrombie ED (1999) Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous L-DOPA: studies in intact and 6-hydroxydopamine-treated rats. J Neurochem 72:1516–1522
Sarre S, De Klippel N, Herregodts P, Ebinger G, Michotte Y (1994) Biotransformation of locally L-dopa in the corpus striatum of the hemi-parkinsonian rats studies with microdialysis. Naunyn Schmiedebergs Arch Pharmacol 350:15–21
Bunney BS, Aghajanian GK, Roth RH (1973) Comparison of effects of L-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurons. Nat New Biol 245:123–125
Mercuri NB, Calabresi P, Bernardi G (1990) Responses of rat substantia nigra compacta neurones to L-DOPA. Br J Pharmacol 100:257–260
Harden DG, Grace AA (1995) Activation of dopamine cell firing by repeated L-DOPA administration to dopamine-depleted rats: its potential role in mediating the therapeutic response to L-DOPA treatment. J Neurosci 15:6157–6166
Maeda T, Kannari K, Suda T, Matsunaga M (1999) Loss of regulation by presynaptic dopamine D2 receptors of exogenous L-DOPA-derived dopamine release in the dopaminergic denervated striatum. Brain Res 817:185–191
Lloyd K, Hornykiewicz O (1970) Parkinson’s disease: activity of L-dopa decarboxylase in discrete brain regions. Science 170:1212–1213
Kannari K, Tanaka H, Maeda T, Tomiyama M, Suda T, Matsunaga M (2000) Reserpine pretreatment prevents increases in extracellular striatal dopamine following L-DOPA administration in rats with nigrostriatal denervation. J Neurochem 74:263–269
Lee WY, Chang JW, Nemeth NL, Kang UJ (1999) Vesicular monoamine transporter-2 and aromatic L-amino acid decarboxylase enhance dopamine delivery after L-3,4-dihydroxyphenylalanine administration in parkinsonian rats. J Neurosci 19:3266–3274
Arai R, Karasawa N, Nagatsu I (1995) L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett 195:195–198
Ng LK, Chase TN, Colburn RW, Kopin IJ (1970) L-Dopa-induced release of cerebral monoamines. Science 170:76–77
Tison F, Mons N, Geffard M, Henry P (1991) The metabolism of exogenous L-dopa in the brain: an immunohistochemical study of its conversion to dopamine in non-catecholaminergic cells of the rat brain. J Neural Transm Park Dis Dement Sect 3:27–39
Yamada H, Aimi Y, Nagatsu I, Taki K, Kudo M, Arai R (2007) Immunohistochemical detection of L-DOPA-derived dopamine within serotonergic fibers in the striatum and the substantia nigra pars reticulate in parkinsonian model rats. Neurosci Res 59:1–7
Arai R, Karazawa N, Nagatsu I (1996) Aromatic L-amino acid decarboxylase is present in serotonergic fibers of the striatum of the rat. A double-labeling immunofluorescence study. Brain Res 706:177–179
Ng LK, Chase TN, Colburn RW, Kopin IJ (1972) L-Dopa in Parkinsonism. A possible mechanism of action. Neurology 22:688–696
Ng LK, Colburn RW, Kopin IJ (1972) Effects of L-dopa on accumulation and efflux of monoamines in particles of brain homogenates. J Pharmacol Exp Ther 183:316–325
Navailles S, Bioulac B, Gross C, De Deurwaerdère P (2010) Serotonergic neurons mediate ectopic release of dopamine induced by L-DOPA in a rat model of Parkinson’s disease. Neurobiol Dis 38:136–143
Tanaka H, Kannari K, Maeda T, Tomiyama M, Suda T, Matsunaga M (1999) Role of serotonergic neurons in L-DOPA-derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. Neuroreport 10:631–634
Kannari K, Yamato H, Shen H, Tomiyama M, Suda T, Matsunaga M (2001) Activation of 5-HT(1A) but not 5-HT(1B) receptors attenuates an increase in extracellular dopamine derived from exogenously administered L-DOPA in the striatum with nigrostriatal denervation. J Neurochem 76:1346–1353
Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA (2010) L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 112:1465–1476
Yamato H, Kannari K, Shen H, Suda T, Matsunaga M (2001) Fluoxetine reduces L-DOPA-derived extracellular DA in the 6-OHDA-lesioned rat striatum. Neuroreport 12:1123–1126
Azmitia EC, Segal M (1978) An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 179:641–667
Navailles S, Bioulac B, Gross C, De Deurwaerdère P (2011) Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson’s disease. Neurobiol Dis 41:585–590
Seeman P (1980) Brain dopamine receptors. Pharmacol Rev 32:229–313
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminal sis the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Navailles S, De Deurwaerdère P (2012) Imbalanced dopaminergic transmission mediated by serotonergic neurons in L-DOPA-induced dyskinesia. Parkinson’s Disease. doi:10.1155/2012/323686
Bartholini G, Da Prada M, Pletscher A (1968) Decrease of cerebral 5-hydroxytryptamine by 3,4-dihydroxyphenylalanine after inhibition of extracerebral decarboxylase. J Pharm Pharmacol 20:228–229
Everett GM, Borcherding JW (1970) L-DOPA: effect on concentrations of dopamine, norepinephrine, and serotonin in brains of mice. Science 168:849–850
Tohgi H, Abe T, Takahashi S, Takahashi J, Hamato H (1993) Alterations in the concentration of serotonergic and dopaminergic substances in the cerebrospinal fluid of patients with Parkinson’s disease, and their changes after L-dopa administration. Neurosci Lett 159:135–138
Gil S, Park C, Lee J, Koh H (2010) The roles of striatal serotonin and L: -amino-acid decarboxylase on L: -DOPA-induced dyskinesia in a hemiparkinsonian rat model. Cell Mol Neurobiol 30:817–825
Héry F, Simonnet G, Bourgoin S, Soubrié P, Artaud F, Hamon M, Glowinski J (1979) Effect of nerve activity on the in vivo release of [3H]serotonin continuously formed from L-[3H]tryptophan in the caudate nucleus of the cat. Brain Res 169:317–334
Thorré K, Sarre S, Smolders I, Ebinger G, Michotte Y (1998) Dopaminergic regulation of serotonin release in the substantia nigra of the freely moving rat using microdialysis. Brain Res 796:107–116
Navailles S, Carta M, Guthrie M, De Deurwaerdère P (2012) L-DOPA and serotonergic neurons: functional implication and therapeutic perspectives in Parkinson’s disease. CNS Agents Med Chem (in press)
Navailles S, De Deurwaerdère P (2011) Presynaptic control of serotonin on striatal dopamine function. Psychopharmacology (Berl) 213:213–242
Gil SJ, Park CH, Lee JE, Minn YK, Koh HC (2011) Positive association between striatal serotonin level and abnormal involuntary movements in chronic L-DOPA-treated hemiparkinsonian rats. Brain Res Bull 84:151–156
Lundblad M, af Bjerkén S, Cenci MA, Pomerleau F, Gerhardt GA, Strömberg I (2009) Chronic intermittent L-DOPA treatment induces changes in dopamine release. J Neurochem 108:998–1008
Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Snadmann-Kell D, Rub U (2002) Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol 249(Suppl 3):III/1-5
Pavese N, Simpson BS, Metta V, Ramlackhansingh A, Chaudhuri KR, Brooks DJ (2011) [(18)F]FDOPA uptake in the raphe nuclei complex reflects serotonin transporter availability. A combined [(18)F]FDOPA and [(11)C]DASB PET study in Parkinson’s disease. Neuroimage. doi:10.1016/j.neuroimage.2011.09.034.
Alexander T, Sortwell CE, Sladek CD, Roth RH, Steece-Collier K (1997) Comparison of neurotoxicity following repeated administration of l-dopa, d-dopa and dopamine to embryonic mesencephalic dopamine neurons in cultures derived from Fisher 344 and Sprague-Dawley donors. Cell Transplant 6:309–315
Basma AN, Morris EJ, Nicklas WJ, Geller HM (1995) L-Dopa cytotoxicity to PC12 cells in culture is via its autoxidation. J Neurochem 64:825–832
Cheng N, Maeda T, Kume T, Kaneko S, Kochiyama H, Akaike A, Goshima Y, Misu Y (1996) Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res 743:278–283
Pardo B, Mean MA, de Yebenes JG (1995) L-Dopa inhibits complex IV of the electron transport chain in catecholamine-rich human neuroblastoma NB69 cells. J Neurochem 64:576–582
Hashiguti H, Nakahara D, Maruyama W, Naoi M, Ikeda T (1993) Simultaneous determination of in vivo hydroxylation of tyrosine and tryptophan in rat striatum by microdialysis-HPLC: relationship between dopamine and serotonin biosynthesis. J Neural Transm Gen Sect 93:213–223
Kuhn DM, Arthur R Jr (1998) Dopamine inactivates tryptophan hydroxylase and forms a redox-cycling quinoprotein: possible endogenous toxin to serotonin neurons. J Neurosci 18:7111–7117
Kuhn DM, Arthur RE Jr (1999) L-DOPA-quinone inactivates tryptophan hydroxylase and converts the enzyme to a redox-cycling quinoprotein. Brain Res Mol Brain Res 73:78–84
Maruyama W, Naoi M, Takahashi A, Watanabe H, Konagaya Y, Mokuno K, Hasegawa S, Nakahara D (1992) The mechanism of perturbation in monoamine metabolism by L-dopa therapy: in vivo and in vitro studies. J Neural Transm Gen Sect 90:183–197
Eskow Jaunajars KL, Dupre KB, Ostock CY, Button T, Deak T, Bishop C (2010) Behavioral and neurochemical effects of chronic L-DOPA treatment on nonmotor sequelae in the hemiparkinsonian rat. Behav Pharmacol 21:627–637
Cao J, Liu J, Zhang QJ, Wang T, Wang S, Han LN, Li Q (2007) The selective 5-HT1A receptor antagonist WAY-100635 inhibits neuronal activity of the ventromedial prefrontal cortex in a rodent model of Parkinson’s disease. Neurosci Bull 23:315–322
De Deurwaerdère P, Chesselet MF (2000) Nigrostriatal lesions alter oral dyskinesia and c-Fos expression induced by the serotonin agonist 1-(m-chlorophenyl)piperazine in adult rats. J Neurosci 20:5170–5178
De Deurwaerdère P, Mignon L, Chesselet MF (2010) Physiological and pathophysiological aspects of 5-HT2C receptors in basal ganglia. In: Di Giovanni G, Neve K (eds) The pathophysiology of central 5-HT2C receptors, vol The receptors series. Springer, New York
Fox SH, Moser B, Brotchie JM (1998) Behavioral effects of 5-HT2C receptor antagonism in the substantia nigra zona reticulata of the 6-hydroxydopamine-lseioned rat model of Parkinson’s disease. Exp Neurol 151:35–49
Fox SH, Brotchie JM (2000) 5-HT2C receptor binding is increased in the substantia nigra pars reticulata in Parkinson’s disease. Mov Disord 15:1046–1049
Gui ZH, Zhang QJ, Liu J, Ali U, Wang Y, Wang T, Chen L, Hou C, Fan LL (2010) In vivo modulation of the firing activity of putative slow- and fast-spiking interneurons in the medial prefrontal cortex by 5-HT3 receptors in 6-hydroxydopamine-minduced parkinsonian rats. Neuroscience 169:1315–1325
Chen CP, Alder JT, Bray L, Kingsbury AE, Francis PT, Foster OJ (1998) Post-synaptic 5-HT1A and 5-HT2A receptors are increased in Parkinson’s disease neocortex. Ann NY Acad Sci 861:288–289
Frechilla D, Cobreros A, Salside L, Moratalla R, Insausti R, Luquin M, Del Rio J (2001) Serotonin 5-HT(1A) receptor expression is selectively enhanced in the striosomal compartment of chronic parkinsonian monkeys. Synapse 39:288–296
Huot P, Johnston TH, Koprich JB, Winkelmolen L, Fox SH, Brotchie JM (2010) Regulation of cortical and striatal 5-HT(1A) receptors in the MPTP-lesioned macaque Neurobiol Aging, in press.
Castro ME, Pascual J, Romon T, Berciano J, Figols J, Pazos A (1998) 5-HT1B receptor binding in degenerative movement disorders. Brain Res 290:323–328
Quirion R, Richard J (1987) Differential effects of selective lesion of cholinergic and dopaminergic neurons on serotonin-type 1 receptors in rat brain. Synapse 1:124–130
Zhang X, Andren PE, Greengard P, Svenningsson P (2008) Evidence for a role of the 5-HT1B receptor and its adaptor protein, p11, in L-DOPA treatment of an animal model of Parkinsonism. Proc Natl Acad Sci U S A 105:2163–2168
Zhang QJ, Wang S, Liu J, Ali U, Gui ZH, Wu ZH, Hui YP, Wang Y, Chen L (2010) Unilateral lesion of the nigrostriatal pathway decreases the response of interneurons in medial prefrontal cortex to 5-HT 2A/2C receptor stimulation in the rat. Brain Res 1312:127–137
Zhang X, Andren PE, Svenningsson P (2007) Changes on 5-HT2 receptor mRNAs in striatum and subthalamic nucleus in Parkinson’s disease model. Physiol Behav 92:29–33
Li Y, Huang XF, Deng C, Meyer B, Wu A, Yu Y, Ying W, Yang GY, Yenari MA, Wang Q (2010) Alterations in 5-HT2A receptor binding in various brain regions among 6-hydroxydopamine-induced parkinsonian rats. Synapse 64:224–230
Riahi G, Morissette M, Parent M, Di Paolo T (2011) Brain 5-HT(2A) receptors in MPTP monkeys and levodopa-induced dyskinesias. Eur J Neurosci 33:1823–1831
Fox SH, Brotchie JM (2000) 5-HT(2C) receptor antagonists enhance the behavioural response to dopamine D(1) receptor agonists in 6-hydroxydopamine-lesioned rat. Eur J Pharmacol 398:59–64
Zhang QJ, Li LB, Niu XL, Liu J, Gui ZH, Feng JJ, Ali U, Hui YP, Wu ZH (2011) The pyramidal neurons in the medial prefrontal cortex show decreased response to 5-hydroxytryptamine-3 receptor stimulation in a rodent model of Parkinson’s disease. Brain Res 1384:69–79
Di Giovanni G, Di Matteo V, Pierucci M, Benigno A, Esposito E (2006) Serotonin involvement in the basal ganglia pathophysiology: could the 5-HT2C receptor be a new target for therapeutic strategies? Curr Med Chem 13:3069–3081
Di Matteo V, Pierucci M, Esposito E, Cresimanno G, Benigno A, Di Giovanni G (2008) Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson’s disease and other motor disorders. Prog Brain Res 172:423–463
Durif F, Vidailhet M, Assal F, Roche C, Bonnet AM, Agid Y (1997) Low-dose clozapine improves dyskinesias in Parkinson’s disease. Neurology 48:658–662
Ikeguchi K, Kuroda A (1995) Mianserin treatment of patients with psychosis induced by antiparkinsonian drugs. Eur Arch Psychiatry Clin Neurosci 244:320–324
Nicholson SL, Brotchie JM (2002) 5-hydroxytryptamine (5-HT, serotonin) and Parkinson’s disease—opportunities for novel therapeutics to reduce the problems of levodopa therapy. Eur J Neurol S3:1–6
Zoldan J, Friedberg G, Livneh M, Melamed E (1995) Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology 45:1305–1308
Zoldan J, Friedberg G, Weizman A, Melamed E (1996) Ondansetron, a 5-HT3 antagonist for visual hallucinations and paranoid delusional disorder associated with chronic l-DOPA therapy in advanced Parkinson’s disease. Adv Neurol 69:541–544
Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH (2010) Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol 67:416–421
Meltzer HY, Mills R, Revell S, Williams H, Johnson A, Bahr D, Friedman JH (2010) Pimavanserin, a serotonin(2A) receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology 35:881–892
Rabey JM (2009) Hallucinations and psychosis in Parkinson’s disease. Parkinsonism Relat Disord 15:S105–S110
Weiner DM, Vanover KE, Brann MR, Meltzer HY, Davis RE (2003) Psychosis of Parkinson’s disease: serotonin 2A inverse agonists as potential therapeutics. Curr Opin Investig Drugs 4:815–819
Meco G, Stirpe P, Edito F, Purcaro C, Valente M, Bernardi S, Vanacore N (2009) Aripiprazole in L-dopa-induced dyskinesias: a one-year open-label pilot study. J Neural Transm 116:881–884
Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, Davis RE, Chase TN, Salamone JD (2008) A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav 90:540–544
Ferguson MC, Nayyar T, Deutch AY, Ansah TA (2010) 5-HT2A receptor antagonists improve motor impairments in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 59:31–36
Werneck AL, Rosso AL, Vincent MB (2009) The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’s disease. Arq Neuropsiquiatr 67:407–412
Rylander D, Parent M, O’Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA (2010) Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol 68:619–628
Zeng BY, Iravani MM, Jackson MJ, Rose S, Parent A, Jenner P (2010) Morphological changes in serotoninergic neurites in the striatum and globus pallidus in levodopa primed MPTP treated common marmosets with dyskinesia. Neurobiol Dis 40:599–607
Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, Bloch B (2009) Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci 29:4829–4835
Picconi B, Pisani A, Barone I, Bonsi P, Centonze D, Bernardi G, Calabresi P (2005) Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology 26:779–783
Picconi B, Ghiglieri V, Calabresi P (2010) L-3,4-Dihydroxyphenylalanine-indued sprouting of serotonin axon terminals: a useful biomarker for dyskinesias? Ann Neurol 68:578–580
Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD (2009) Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson’s disease patients. Brain 132:309–318
Krack P, Hamel W, Mehdorn HM, Deuschl G (1999) Surgical treatment of Parkinson’s disease. Curr Opin Neurol 12:417–425
Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL (1998) Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 339:1105–1111
Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR (2000) Pathophysiologic basis of surgery for Parkinson’s disease. Neurology 55(Suppl 6):S7–S12
Welter ML, Houeto JL, Tezenas du Montcel S, Mesnage V, Bonnet AM, Pillon B, Arnulf I, Pidoux B, Dormont D, Cornu P, Agid Y (2002) Clinical predictive factors of subthalamic stimulation in Parkinson’s disease. Brain 125:575–583
Benabid AL, Chabardes S, Seigneuret E, Pollak P, Fraix V, Krack P, Lebas JF, Grand S, Piallat B (2005) Functional neurosurgery: past, present, and future. Clin Neurosurg 52:265–270
Fraix V, Pollak P, Van Blercom N, Xie J, Krack P, Koudsie A, Benabid AL (2000) Effect of subthalamic nucleus stimulation on levodopa-induced dyskinesia in Parkinson’s disease. Neurology 26:1921–1923
Krack P, Limousin P, Benabid AL, Pollak P (1997) Chronic stimulation of subthalamic nucleus improves levodopa-induced dyskinesia in Parkinson’s disease. Lancet 350:1676
Krack P, Batir A, Van Blercom N et al (2003) Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med 349:1925–1934
Benabid AL, Benazzouz A, Pollak P (2002) Mechanisms of deep brain stimulation. Mov Disord 17(Suppl 3):S73–S74
Dostrovsky JO, Lozano AM (2002) Mechanisms of deep brain stimulation. Mov Disord 17(Suppl 3):S63–S68
McIntyre CC, Savasta M, GoffL Kerkerian-Le, Vitek JL (2004) Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115:1239–1248
Lopiano L, Rizzone M, Bergamasco B, Tavella A, Torre E, Perozzo P, Valentini MC, Lanotte M (2001) Deep brain stimulation of the subthalamic nucleus: clinical effectiveness and safety. Neurology 27:552–554
Molinuevo JL, Valldeoriola F, Tolosa E, Rumia J, Valls-Sole J, Roldan H, Ferrer E (2000) Levodopa withdrawal after bilateral subthalamic nucleus stimulation in advanced Parkinson disease. Arch Neurol 57:983–988
Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A (1999) Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology 53:85–90
Oueslati A, Sgambato-Faure V, Melon C, Kachidian P, Gubellini P, Amri M, Kerkerian-Le Goff L, Salin P (2007) High-frequency stimulation of the subthalamic nucleus potentiates L-DOPA-induced neurochemical changes in the striatum in a rat model of Parkinson’s disease. J Neurosci 27:2377–2386
Simonin C, Tir M, Devos D, Jreisler A, Dujardin K, Salleron J, Delval A, Blond S, Defebvre L, Destee A, Krystkowiak P (2009) Reduced levodopa-induced complications after 5 years of subthalamic stimulation in Parkinson’s disease: a second honeymoon. J Neurol 256:1736–1741
Gubellini P, Eusebio A, Oueslati A, Melon C, Kerkerian-Le Goff L, Salin P (2006) Chronic high-frequency stimulation of the subthalamic nucleus and L-DOPA treatment in experimental parkinsonism: effects on motor behaviour and striatal glutamate transmission. Eur J Neurosci 24:1802–1814
Nimura T, Yamaguchi K, Ando T, Shibuya S, Oikawa T, Nakagawa A, Shirane R, Itoh M, Tominaga T (2005) Attenuation of fluctuating striatal synaptic dopamine levels in patients with Parkinson disease in response to subthalamic nucleus stimulation: a positron emission tomography study. J Neurosurg 103:968–973
Lacombe E, Carcenac C, Boulet S, Feurstein C, Bertrand A, Poupard A, Savasta M (2007) High-frequency stimulation of the subthalamic nucleus prolongs the increase in striatal dopamine induced by acute l-3,4-dihydroxyphenylalanine in dopaminergic denervated rats. Eur J Neurosci 26:1670–1680
Zesiewicz TA, Hauser RA (2002) Depression in Parkinson’s disease. Curr Psychiatry Rep 4:69–73
Nieoullon A (2002) Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83
Siri C, Cilia R, De Gaspari D, Canesi M, Meucci N, Zecchinelli AL, Pezzoli G, Antonini A (2010) Cognitive status of patients with Parkinson’s disease and pathological gambling. J Neurol 257:247–252
Voon V, Fernagut PO, Wickens J, Baunez C, Rodriguez M, Pavon N, Juncos JL, Obeso JA, Bezard E (2009) Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol 8:1140–1149
Acknowledgments
This work was supported by grants from “Centre National de la Recherche Scientifique”, Bordeaux 2 University and the Fondation de France. The authors thank Dr Martin Guthrie for linguistic assistance.
Conflict of Interest
The authors report no biomedical financial interest or potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navailles, S., De Deurwaerdère, P. Contribution of Serotonergic Transmission to the Motor and Cognitive Effects of High-Frequency Stimulation of the Subthalamic Nucleus or Levodopa in Parkinson’s Disease. Mol Neurobiol 45, 173–185 (2012). https://doi.org/10.1007/s12035-011-8230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-011-8230-0