Abstract
Parkinson’s disease (PD) is pathologically characterized by intracellular α-synuclein-rich protein aggregates, named Lewy bodies (LB), and by the progressive loss of dopaminergic neurons in the substantia nigra. Several heavy metals, including zinc (Zn), have been suggested to play a role in PD progression, although the exact role of Zn in neurodegeneration remains to be fully elucidated. To address this gap, we investigated the effects of Zn modulation on the progression of degeneration in mice injected with PD patient-derived LB-extracts carrying toxic α-synuclein aggregates. Zn modulation was achieved using either a clioquinol-enriched diet, a Zn ionophore that redistributes cellular Zn, or a Zn-enriched diet that increases Zn levels. Clioquinol treatment significantly prevented dopaminergic neurodegeneration and reduced α-synuclein-associated pathology in LB-injected mice, while no differences were observed with Zn supplementation. Biochemical analyses further demonstrate that the expression levels of vesicle-specific Zn transporter ZnT3 in the striatum of LB-injected mice treated with clioquinol were decreased, suggesting an intracellular redistribution of Zn. Additionally, we found that clioquinol modulates the autophagy-lysosomal pathway by enhancing lysosomal redistribution within the neuronal compartments. Collectively, we found that in vivo pharmacological chelation of Zn, by dampening Zn-mediated cytotoxicity, can result in an overall attenuation of PD-linked lysosomal alterations and dopaminergic neurodegeneration. The results support zinc chelation as a disease-modifying strategy for treating PD.





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in Table 1 of supplementary data; further inquiries can be directed to the corresponding author.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALP:
-
Autophagy-lysosomal pathway
- ClQ:
-
Clioquinol
- CNS:
-
Central nervous system
- ELISA:
-
Enzyme-linked immunosorbent assay
- PD:
-
Parkinson’s disease
- α-syn:
-
α-Synuclein
- LB:
-
Lewy bodies
- LC3:
-
MAP1LC3B, microtubule-associated protein 1 light chain 3 β
- MTF1:
-
Metal transcription factor 1
- PK:
-
Proteinase K
- SNpc:
-
Substantia nigra pars compacta
- SOD1:
-
Superoxide dismutase 1
- TH:
-
Tyrosine hydroxylase
- Zn:
-
Zinc
References
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376. https://doi.org/10.1136/jnnp.2007.131045
Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, Singleton A, Olanow CW et al (2015) Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14:855–866. https://doi.org/10.1016/s1474-4422(15)00006-x
Bocca B, Alimonti A, Senofonte O, Pino A, Violante N, Petrucci F, Sancesario G, Forte G (2006) Metal changes in CSF and peripheral compartments of parkinsonian patients. J Neurol Sci 248:23–30. https://doi.org/10.1016/j.jns.2006.05.007
Gardner B, Dieriks BV, Cameron S, Mendis LHS, Turner C, Faull RLM, Curtis MA (2017) Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Sci Rep 7:10454. https://doi.org/10.1038/s41598-017-10659-6
Genoud S, Roberts BR, Gunn AP, Halliday GM, Lewis SJG, Ball HJ, Hare DJ, Double KL (2017) Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 9:1447–1455. https://doi.org/10.1039/c7mt00244k
Mezzaroba L, Alfieri DF, Colado Simao AN, Vissoci Reiche EM (2019) The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 74:230–241. https://doi.org/10.1016/j.neuro.2019.07.007
Marger L, Schubert CR, Bertrand D (2014) Zinc: an underappreciated modulatory factor of brain function. Biochem Pharmacol 91:426–435. https://doi.org/10.1016/j.bcp.2014.08.002
Hwang JJ, Kim HN, Kim J, Cho DH, Kim MJ, Kim YS, Kim Y, Park SJ et al (2010) Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals 23:997–1013. https://doi.org/10.1007/s10534-010-9346-9
Liuzzi JP, Yoo C (2013) Role of zinc in the regulation of autophagy during ethanol exposure in human hepatoma cells. Biol Trace Elem Res 156:350–356. https://doi.org/10.1007/s12011-013-9816-3
Kambe T, Tsuji T, Hashimoto A, Itsumura N (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev 95:749–784. https://doi.org/10.1152/physrev.00035.2014
Adlard PA, Parncutt JM, Finkelstein DI, Bush AI (2010) Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci 30:1631–1636. https://doi.org/10.1523/JNEUROSCI.5255-09.2010
Sikora J, Kieffer BL, Paoletti P, Ouagazzal AM (2020) Synaptic zinc contributes to motor and cognitive deficits in 6-hydroxydopamine mouse models of Parkinson’s disease. Neurobiol Dis 134:104681. https://doi.org/10.1016/j.nbd.2019.104681
Bourdenx M, Nioche A, Dovero S, Arotcarena ML, Camus S, Porras G, Thiolat ML, Rougier NP et al (2020) Identification of distinct pathological signatures induced by patient-derived alpha-synuclein structures in nonhuman primates. Sci Adv 6:eaaz9165. https://doi.org/10.1126/sciadv.aaz9165
Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, Vital A, Vila M et al (2012) Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A 109:9611–9616. https://doi.org/10.1073/pnas.1112368109
Murphy KE, Cottle L, Gysbers AM, Cooper AA, Halliday GM (2013) ATP13A2 (PARK9) protein levels are reduced in brain tissue of cases with Lewy bodies. Acta Neuropathol Commun 1:11–11. https://doi.org/10.1186/2051-5960-1-11
Tsunemi T, Krainc D (2014) Zn(2)(+) dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum Mol Genet 23:2791–2801. https://doi.org/10.1093/hmg/ddt572
Tsunemi T, Perez-Rosello T, Ishiguro Y, Yoroisaka A, Jeon S, Hamada K, Krishna Vangipuram Suresh M, Wong YC et al (2019) Increased lysosomal exocytosis induced by lysosomal Ca(2+) channel agonists protects human dopaminergic neurons from alpha-synuclein toxicity. J Neurosci. https://doi.org/10.1523/JNEUROSCI.3085-18.2019
Tsunemi T, Hamada K, Krainc D (2014) ATP13A2/PARK9 regulates secretion of exosomes and alpha-synuclein. J Neurosci 34:15281–15287. https://doi.org/10.1523/JNEUROSCI.1629-14.2014
Gonzalez N, Arcos-Lopez T, Konig A, Quintanar L, Menacho Marquez M, Outeiro TF, Fernandez CO (2019) Effects of alpha-synuclein post-translational modifications on metal binding. J Neurochem 150:507–521. https://doi.org/10.1111/jnc.14721
Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G et al (2014) Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal 21:195–210. https://doi.org/10.1089/ars.2013.5593
Moreau C, Duce JA, Rascol O, Devedjian JC, Berg D, Dexter D, Cabantchik ZI, Bush AI et al (2018) Iron as a therapeutic target for Parkinson’s disease. Mov Disord 33:568–574. https://doi.org/10.1002/mds.27275
Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM (2009) Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats. Neurotoxicology 30:240–248. https://doi.org/10.1016/j.neuro.2008.12.007
Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I et al (2001) Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 30:665–676. https://doi.org/10.1016/s0896-6273(01)00317-8
Finkelstein DI, Hare DJ, Billings JL, Sedjahtera A, Nurjono M, Arthofer E, George S, Culvenor JG et al (2016) Clioquinol improves cognitive, motor function, and microanatomy of the alpha-synuclein hA53T transgenic mice. ACS Chem Neurosci 7:119–129. https://doi.org/10.1021/acschemneuro.5b00253
Lei P, Ayton S, Appukuttan AT, Volitakis I, Adlard PA, Finkelstein DI, Bush AI (2015) Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol Dis 81:168–175. https://doi.org/10.1016/j.nbd.2015.03.015
Nguyen T, Hamby A, Massa SM (2005) Clioquinol down-regulates mutant huntingtin expression in vitro and mitigates pathology in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A 102:11840–11845. https://doi.org/10.1073/pnas.0502177102
Shi L, Huang C, Luo Q, Xia Y, Liu W, Zeng W, Cheng A, Shi R et al (2020) Clioquinol improves motor and non-motor deficits in MPTP-induced monkey model of Parkinson’s disease through AKT/mTOR pathway. Aging (Albany NY) 12:9515–9533. https://doi.org/10.18632/aging.103225
Soria FN, Paviolo C, Doudnikoff E, Arotcarena ML, Lee A, Danne N, Mandal AK, Gosset P et al (2020) Synucleinopathy alters nanoscale organization and diffusion in the brain extracellular space through hyaluronan remodeling. Nat Commun 11:3440. https://doi.org/10.1038/s41467-020-17328-9
Recasens A, Dehay B, Bove J, Carballo-Carbajal I, Dovero S, Perez-Villalba A, Fernagut PO, Blesa J et al (2014) Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75:351–362. https://doi.org/10.1002/ana.24066
Teil M, Dovero S, Bourdenx M, Arotcarena ML, Camus S, Porras G, Thiolat ML, Trigo-Damas I et al (2022) Brain injections of glial cytoplasmic inclusions induce a multiple system atrophy-like pathology. Brain 145:1001–1017. https://doi.org/10.1093/brain/awab374
Arotcarena ML, Dovero S, Prigent A, Bourdenx M, Camus S, Porras G, Thiolat ML, Tasselli M et al (2020) Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 143:1462–1475. https://doi.org/10.1093/brain/awaa096
Mosselmans JF, Quinn PD, Dent AJ, Cavill SA, Moreno SD, Peach A, Leicester PJ, Keylock SJ et al (2009) I18–the microfocus spectroscopy beamline at the Diamond Light Source. J Synchrotron Radiat 16:818–824. https://doi.org/10.1107/S0909049509032282
Solé VA, Papillon E, Cotte M, Walter P, Susini J (2007) A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectrochim Acta B 62:63–68. https://doi.org/10.1016/j.sab.2006.12.002
Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A (2019) Moving beyond P values: data analysis with estimation graphics. Nat Methods 16:565–566. https://doi.org/10.1038/s41592-019-0470-3
Ding WQ, Lind SE (2009) Metal ionophores - an emerging class of anticancer drugs. IUBMB Life 61:1013–1018. https://doi.org/10.1002/iub.253
Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE (2005) Anticancer activity of the antibiotic clioquinol. Cancer Res 65:3389–3395. https://doi.org/10.1158/0008-5472.CAN-04-3577
Mao X, Schimmer AD (2008) The toxicology of Clioquinol. Toxicol Lett 182:1–6. https://doi.org/10.1016/j.toxlet.2008.08.015
Bengoa-Vergniory N, Faggiani E, Ramos-Gonzalez P, Kirkiz E, Connor-Robson N, Brown LV, Siddique I, Li Z et al (2020) CLR01 protects dopaminergic neurons in vitro and in mouse models of Parkinson’s disease. Nat Commun 11:4885. https://doi.org/10.1038/s41467-020-18689-x
Trist BG, Hilton JB, Hare DJ, Crouch PJ, Double KL (2021) Superoxide dismutase 1 in health and disease: how a frontline antioxidant becomes neurotoxic. Angew Chem Int Ed Engl 60:9215–9246. https://doi.org/10.1002/anie.202000451
Trist BG, Davies KM, Cottam V, Genoud S, Ortega R, Roudeau S, Carmona A, De Silva K et al (2017) Amyotrophic lateral sclerosis-like superoxide dismutase 1 proteinopathy is associated with neuronal loss in Parkinson’s disease brain. Acta Neuropathol 134:113–127. https://doi.org/10.1007/s00401-017-1726-6
Arotcarena ML, Soria FN, Cunha A, Doudnikoff E, Prevot G, Daniel J, Blanchard-Desce M, Barthelemy P et al (2022) Acidic nanoparticles protect against alpha-synuclein-induced neurodegeneration through the restoration of lysosomal function. Aging Cell 21:e13584. https://doi.org/10.1111/acel.13584
Pu J, Guardia CM, Keren-Kaplan T, Bonifacino JS (2016) Mechanisms and functions of lysosome positioning. J Cell Sci 129:4329–4339. https://doi.org/10.1242/jcs.196287
Johnson DE, Ostrowski P, Jaumouille V, Grinstein S (2016) The position of lysosomes within the cell determines their luminal pH. J Cell Biol 212:677–692. https://doi.org/10.1083/jcb.201507112
Kumar V, Singh D, Singh BK, Singh S, Mittra N, Jha RR, Patel DK, Singh C (2018) Alpha-synuclein aggregation, Ubiquitin proteasome system impairment, and L-Dopa response in zinc-induced Parkinsonism: resemblance to sporadic Parkinson’s disease. Mol Cell Biochem 444:149–160. https://doi.org/10.1007/s11010-017-3239-y
Krall RF, Tzounopoulos T, Aizenman E (2021) The function and regulation of zinc in the brain. Neuroscience 457:235–258. https://doi.org/10.1016/j.neuroscience.2021.01.010
Dos Santos AB, Kohlmeier KA, Rocha ME, Barreto GE, Barreto JA, de Souza ACA, Bezerra MA (2018) Hair in Parkinson’s disease patients exhibits differences in Calcium, Iron and Zinc concentrations measured by flame atomic absorption spectrometry - FAAS. J Trace Elem Med Biol 47:134–139. https://doi.org/10.1016/j.jtemb.2018.02.003
Tarohda T, Ishida Y, Kawai K, Yamamoto M, Amano R (2005) Regional distributions of manganese, iron, copper, and zinc in the brains of 6-hydroxydopamine-induced parkinsonian rats. Anal Bioanal Chem 383:224–234. https://doi.org/10.1007/s00216-005-3423-x
Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD (1989) Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 52:1830–1836. https://doi.org/10.1111/j.1471-4159.1989.tb07264.x
Lippi SLP, Craven KM, Hernandez CM, Grant GM, Flinn JM (2019) Perfusion alters free zinc levels in the rodent brain. J Neurosci Methods 315:14–16. https://doi.org/10.1016/j.jneumeth.2018.12.018
Schrag M, Dickson A, Jiffry A, Kirsch D, Vinters HV, Kirsch W (2010) The effect of formalin fixation on the levels of brain transition metals in archived samples. Biometals 23:1123–1127. https://doi.org/10.1007/s10534-010-9359-4
Bezard E, Dehay B (2022) Aggregation and spread of synuclein in Parkinson’s disease. Med Sci (Paris) 38:45–51. https://doi.org/10.1051/medsci/2021241
Blesa J, Foffani G, Dehay B, Bezard E, Obeso JA (2022) Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci 23:115–128. https://doi.org/10.1038/s41583-021-00542-9
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. https://doi.org/10.1038/nrn.2016.178
Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, Santos DP, Blanz J et al (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357:1255–1261. https://doi.org/10.1126/science.aam9080
Zecca L, Pietra R, Goj C, Mecacci C, Radice D, Sabbioni E (1994) Iron and other metals in neuromelanin, substantia nigra, and putamen of human brain. J Neurochem 62:1097–1101. https://doi.org/10.1046/j.1471-4159.1994.62031097.x
Carballo-Carbajal I, Laguna A, Romero-Gimenez J, Cuadros T, Bove J, Martinez-Vicente M, Parent A, Gonzalez-Sepulveda M et al (2019) Brain tyrosinase overexpression implicates age-dependent neuromelanin production in Parkinson’s disease pathogenesis. Nat Commun 10:973. https://doi.org/10.1038/s41467-019-08858-y
Liuzzi JP, Pazos R (2020) Interplay Between Autophagy and Zinc. J Trace Elem Med Biol 62:126636. https://doi.org/10.1016/j.jtemb.2020.126636
Lichtlen P, Schaffner W (2001) The, “metal transcription factor” MTF-1: biological facts and medical implications. Swiss Med Wkly 131:647–652. https://doi.org/10.4414/Smw.2001.09672
Nuttall JR, Oteiza PI (2012) Zinc and the ERK kinases in the developing brain. Neurotox Res 21:128–141. https://doi.org/10.1007/s12640-011-9291-6
Jiao M, Ren F, Zhou L, Zhang X, Zhang L, Wen T, Wei L, Wang X et al (2014) Peroxisome proliferator-activated receptor alpha activation attenuates the inflammatory response to protect the liver from acute failure by promoting the autophagy pathway. Cell Death Dis 5:e1397. https://doi.org/10.1038/cddis.2014.361
Lee JY, Son HJ, Choi JH, Cho E, Kim J, Chung SJ, Hwang O, Koh JY (2009) Cytosolic labile zinc accumulation in degenerating dopaminergic neurons of mouse brain after MPTP treatment. Brain Res 1286:208–214. https://doi.org/10.1016/j.brainres.2009.06.046
Tamano H, Morioka H, Nishio R, Takeuchi A, Takeda A (2018) AMPA-induced extracellular Zn(2+) influx into nigral dopaminergic neurons causes movement disorder in rats. Neurotoxicology 69:23–28. https://doi.org/10.1016/j.neuro.2018.08.008
Tamano H, Nishio R, Morioka H, Takeda A (2019) Extracellular Zn(2+) Influx into Nigral Dopaminergic Neurons Plays a Key Role for Pathogenesis of 6-Hydroxydopamine-Induced Parkinson’s Disease in Rats. Mol Neurobiol 56:435–443. https://doi.org/10.1007/s12035-018-1075-z
Park MH, Lee SJ, Byun HR, Kim Y, Oh YJ, Koh JY, Hwang JJ (2011) Clioquinol induces autophagy in cultured astrocytes and neurons by acting as a zinc ionophore. Neurobiol Dis 42:242–251. https://doi.org/10.1016/j.nbd.2011.01.009
Yu H, Zhou Y, Lind SE, Ding WQ (2009) Clioquinol targets zinc to lysosomes in human cancer cells. Biochem J 417:133–139. https://doi.org/10.1042/BJ20081421
Koh JY, Kim HN, Hwang JJ, Kim YH, Park SE (2019) Lysosomal dysfunction in proteinopathic neurodegenerative disorders: possible therapeutic roles of cAMP and zinc. Mol Brain 12:18. https://doi.org/10.1186/s13041-019-0439-2
Wallings R, Connor-Robson N, Wade-Martins R (2019) LRRK2 interacts with the vacuolar-type H+-ATPase pump a1 subunit to regulate lysosomal function. Hum Mol Genet 28:2696–2710. https://doi.org/10.1093/hmg/ddz088
Carboni E, Tatenhorst L, Tonges L, Barski E, Dambeck V, Bahr M, Lingor P (2017) Deferiprone rescues behavioral deficits induced by mild iron exposure in a mouse model of alpha-synuclein aggregation. Neuromol Med 19:309–321. https://doi.org/10.1007/s12017-017-8447-9
Devos D, Cabantchik ZI, Moreau C, Danel V, Mahoney-Sanchez L, Bouchaoui H, Gouel F, Rolland AS et al (2020) Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J Neural Transm (Vienna). https://doi.org/10.1007/s00702-019-02138-1
Acknowledgements
We thank Guillaume Dabée and Christelle Martin for animal care, as well as Dr. Federico N. Soria for the script to analyze the TH-LAMP2 perinuclear fractions. The synchrotron Diamond Light Source is acknowledged for provision of I18 beam time (exp. SP28279). We would also like to thank Tina Geraki for scientific support. MT was supported by a Ministère de l’Enseignement Supérieur et de la Recherche fellowship (France). The human samples were obtained from the Brain Bank GIE NeuroCEB (BRIF number 0033-00011), funded by France Alzheimer, France Parkinson, ARSEP, and Connaître les Syndromes Cérébelleux, to which we express our gratitude.
Funding
The University of Bordeaux and the Centre National de la Recherche Scientifique provided infrastructural support. This study received financial support from the French government in the framework of the University of Bordeaux’s IdEx “Investments for the Future” program/GPR BRAIN_2030. Animal experiments were performed at the Animal Facilities of the University of Bordeaux, supported by the Région Nouvelle-Aquitaine.
Author information
Authors and Affiliations
Contributions
BD isolated LB fractions. MT performed the surgeries. MT and ED performed the animal termination. MT performed histologic and immunohistochemical analysis of the data. MT and MLT performed the biochemistry experiments. MT, SB, and BD performed the synchrotron analysis. MT prepared the figures. MT, EB, and BD wrote the paper with input from all authors. BD and EB conceptualized the study and designed experiments. EB and BD secured funding.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
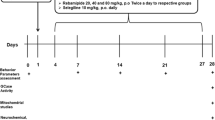
Animals. Experiments were performed following the European Union directive of September 22, 2010 (2010/63/EU) on the protection of animals used for scientific purposes. The Institutional Animal Care and Ethical Committee of Bordeaux University (CE50, France) approved experiments accepted by the ministry under references APAFIS#17592–2018111914281699.
Human tissues. The samples were obtained from brains collected in a Brain Donation Program of the Brain Bank “GIE NeuroCEB” run by a consortium of Patients Associations: ARSEP (association for research on multiple sclerosis), CSC (cerebellar ataxias), France Alzheimer, and France Parkinson. The consent documents were signed by the patients themselves or their next of kin in their name, in accordance with the French Bioethical Laws. The Brain Bank GIE NeuroCEB (Bioresource Research Impact Factor number BB-0033–00011) has been declared at the Ministry of Higher Education and Research and has received approval to distribute samples (agreement AC-2013–1887).
Consent for Publication
Not applicable.
Conflict of Interest
E.B. owns equity stake in Motac Holding Ltd. and receives consultancy payments from Motac Neuroscience Ltd. All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary file2
Supplementary Figure 1: Clioquinol treatment inhibits the loss of neurons in the substantia nigra, but both dopaminergic fiber density and dopamine transporter expression remain unchanged in LB-injected or Vehicle-injected mice. (A-B) Scatter plots of Tyrosine Hydroxylase (TH) and Nissl-positive neuron count in the substantia nigra of Vehicle and LB-injected mice treated with Clioquinol or zinc. (B) Illustrative images (left) and quantifications (right) of TH staining in the whole striatum of mice injected with Vehicle or LB fractions that received one of three diets. (C) Illustrative images (left) and quantifications (right) of DAT staining in the whole striatum of mice injected with Vehicle or LB fractions that received one of three diets. Comparisons were made using two-way ANOVA followed by Tukey’s post-hoc analysis, n=5 per group. Scale bar =500μm. * p<0.05 vs Veh, Veh ClQ, LB ClQ; # p<0.05 vs Veh, Veh Zn. (PNG 2057 kb)

Supplementary file3
Supplementary Figure 2: Expression of monomeric α-synuclein remains unchanged in LB-injected mice treated with Clioquinol and zinc. Immunoblot and quantification of α-synuclein expression in the substantia nigra of injected mice. Scatter plots represent the mean protein expression normalized by β-Actin levels in Vehicle and LB mice treated with clioquinol or zinc. Comparisons were made using two-way ANOVA followed by Tukey’s post-hoc analysis, n=4-5 per group. (PNG 282 kb)

Supplementary file4
Supplementary Figure 3: Zinc Transporter expression is unaltered in the substantia nigra of mice with modulated zinc diets. Representative immunoblots and histograms of ZnT4 (A), ZIP8 (B) and SOD1 (C) expressions in the substantia nigra. Scatter plots represent the mean protein expression normalized by β-Actin levels in Vehicle and LB mice treated with clioquinol or zinc. Comparisons were made using two-way ANOVA followed by Tukey’s post-hoc analysis, n=4-5 per group. (PNG 531 kb)

Supplementary file5
Supplementary Figure 4: Clioquinol and zinc treatments induce few alterations in metal concentrations in the SN of LB-injected mice. Levels of zinc (A), calcium (B), iron (C), copper (D), manganese (E), and sulfur (F) were measured using synchrotron X-ray fluorescence in the ipsilateral SN of control and LB-injected mice treated with clioquinol or zinc. Comparisons were made using two-way ANOVA followed by Tukey’s post-hoc analysis, n=5 per group. * p<0.05 vs all (Zn), vs LB (Ca), vs LB ClQ and LB Zn (Fe), # p<0.05 Veh Zn vs LB and LB ClQ. (PNG 151 kb)

Supplementary file6
Supplementary Figure 5: Clioquinol and zinc induce alterations in expression of autophagy and lysosomal markers. (A-C) Representative images and quantification of LC3-II (A), p62 (B), and LAMP1 (C) immunoblotting in the different experimental groups. Scatter plots represent the mean protein expression normalized by β-Actin levels in Vehicle and LB mice treated with clioquinol or zinc. Comparisons were made using two-way ANOVA followed by Tukey’s post-hoc analysis, n=4-5 per group. * p<0.05 vs Veh, Veh Zn, LB. #p<0.05 vs Veh, LB, LB ClQ. (PNG 745 kb)

Supplementary file7
Supplementary Figure 6: Zinc supplementation induces alterations in protein ubiquitination in mice. (A-C) Representative images (A) and quantification of ubiquitin (B) and poly-ubiquitin (C) immunoblotting in the different experimental groups. Scatter plots represent the mean protein expression normalized by β-Actin levels in Vehicle and LB mice treated with clioquinol or zinc. Comparisons were made using two-way ANOVA followed by Tukey’s post-hoc analysis, n=4 per group. * p<0.05 vs LB ClQ (ClQ), vs Veh, LB, LB Zn (Zn), vs Veh, LB Zn (Zn polyUb); #p<0.05 vs Veh, LB, $p<0.05 vs LB. (PNG 885 kb)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teil, M., Doudnikoff, E., Thiolat, ML. et al. The Zinc Ionophore Clioquinol Reduces Parkinson’s Disease Patient-Derived Brain Extracts-Induced Neurodegeneration. Mol Neurobiol 59, 6245–6259 (2022). https://doi.org/10.1007/s12035-022-02974-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-022-02974-5