Abstract
Purpose
The treatment of recurrent high-grade gliomas (HGG) is controversial. There are different therapeutic schedules but without a clear orientation about which of them should be used in each clinical situation. In addition, when patients suffer a second recurrence or they have poor performance status, they are excluded from clinical trials, although second recurrences and poor performance status are indeed more and more real and common situations in the clinical setting. In this study, we assessed the efficacy and safety of fotemustine (FTM) in HGG [fundamentally, glioblastomas (GB)], independent of time of recurrence or performance status.
Methods/patients
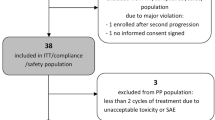
Retrospective study in HGG patients treated with FTM in second or further line according to standard, the Addeo or any other scheme, starting treatment prior to 30 November 2012. Included patients reflect the regular situation in which the drug is used in terms of comorbidities and analytic situation (hematologic, renal and hepatic functions). Response assessment was performed by MRI and according to the clinical protocols of each center (every 8–12 weeks). Clinical situation and supportive care drugs were evaluated in each medical consultation. Clinical end-points analyzed, among others, were: PFS-6, PFS, OS, response rates, toxicity, quality of life and neurocognitive impact.
Results
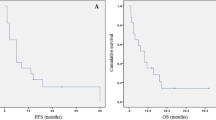
In terms of activity, an overall response rate of 8 % was observed: partial response 6 % (7 patients) and complete response 2 % (2 patients). The median time to achieve the greater response with FTM was 73 days (4–841 days). Patients treated according to the Addeo schedule had a shorter time to greater response in comparison with other schedules (85.9 vs 114 days), although without statistical significance. There were no significant differences in progression-free survival (PFS) when comparing different FTM schedules or using FTM in first or second recurrence. Median PFS: 3 months. PFS-6: 30.3 %. Overall survival (OS): although without significant differences, a tendency to better survival when using the Addeo schedule versus other schedules was observed (at 6 months, 44.6 vs 34.5 %; at 12 months, 25 vs 23.6 %; at 18 months, 11.5 vs 7.9 %), as well as if earlier use (second vs third line) concerning OS-12 (33.7 vs 18.2 %). Median OS: 5.2 months. Grades 3–4 toxicity was 28 % (31 patients), being neutropenia (4 %) and thrombocytopenia (17 %) the most frequent adverse reactions. From quality of life and neuro-cognitive function perspectives, 11 patients (10 %) and 16 (14 %) improved the Karnofsky Index and neurological impairment, respectively, after FTM treatment.
Conclusion
This study has shown that FTM is safe and has a comparable activity with other available therapeutic options of use in the treatment of recurrent HGG.



Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Desjardins A, Quinn JA, Vredenburgh JJ, Sathornsumetee S, Friedman AH, Herndon JE, et al. Phase II study of imatinib mesylate and hydroxiurea for recurrent grade III malignant gliomas. J Neurooncol. 2007;83(1):53–60.
Franceschi E, Cavalo G, Lonardi S, Magrini E, Tosoni A, Grosso D, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer. 2007;96(7):1047–51.
Neyns B, Sadones J, Joosens E, Boutens F, Verbeke L, Baurain JF, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–603.
Prados MD, Lamborn K, Yung WK, Jaeckle K, Robins HI, Mehta M, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8(2):189–93.
Fabrini MG, Silvano G, Lolli I, Perrone F, Marsella A, Scotti V, et al. A multi-institutional phase II study on second line fotemustine chemotherapy in recurrent glioblastoma. J Neurooncol. 2009;92(1):79–86.
Scoccianti S, Detti B, Sardaro A, Iannalfi A, Meattini I, Leonulli BG, et al. Second-line chemotherapy with fotemustine in temozolomide pretreated patients with relapsing glioblastoma: a single institution experience. Anticancer Drugs. 2008;19(6):613–20.
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab plus irinotecan in recurrent glioblastoma mutiforme. J Clin Oncol. 2007;25(30):4722–9.
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40.
Fadul CE, Kingman LS, Meyer LP, Cole BF, Eskey CJ, Rhodes CH, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;90(2):229–35.
Reardon DA, Quinn JA, Vredenburgh J, Rich JN, Gururangan S, Badruddoja M, et al. Phase II trial of irinotecan plus celecoxib in adults with recurrent malignant glioma. Cancer. 2005;103(2):329–38.
de Groot JF, Gilbert MR, Aldape K, Hess KR, Hanna TA, Ictech S, et al. Phase II study of carboplatin and erlotinib in patients with recurrent glioblastoma. J Neuroncol. 2008;90(1):89–97.
Desjardins A, Reardon DA, Herndon JE 2nd, Marcello J, Quinn JA, Rich JN, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2007;14(21):7068–73.
Chamberlain MC, Glantz MJ. CPT-11 for recurrent temozolomide-refractory 1p19q co-deleted anaplastic oligodendroglioma. J Neuroncol. 2008;89(2):231–8.
Fabi A, Metro G, Russillo M, Vidiri A, Carapella CM, Maschio M, et al. Treatment of recurrent malignant gliomas with fotemustine monotherapy: impact of dose and correlation with MGMT promoter methylation. BMC Cancer. 2009;9:101.
Brandes AA, Tosoni A, Franceschi E, Blatt V, Santoro A, Faedi M, et al. Fotemustine as second-line treatment for recurrent or progressive glioblastoma after concomitant and/or adjuvant temozolomide: a phase II trial of Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol. 2009;64(4):769–75.
Addeo R, Caraglia M, De Santi MS, Montella L, Abbruzzese A, Parlato C, et al. A new schedule of fotemustine in temozolomide-pretreated patients with relapsing glioblastoma. J Neurooncol. 2011;102(3):417–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Informed consent
The protocol was approved by research ethics committees from each participating center.
Research involving human participants and/or animals
We have fulfilled the local laws about human research.
Additional information
On behalf the Spanish Group of Investigation in Neurooncology (GEINO).
Rights and permissions
About this article
Cite this article
Pérez-Segura, P., Manneh, R., Ceballos, I. et al. GEINOFOTE: efficacy and safety of fotemustine in patients with high-grade recurrent gliomas and poor performance status. Clin Transl Oncol 18, 805–812 (2016). https://doi.org/10.1007/s12094-015-1444-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-015-1444-2