Abstract
Background/Aim
First-line bevacizumab-based therapies have been shown to improve clinical outcomes in patients with non-squamous non-small-cell lung cancer (NSCLC). We aimed to descriptively analyse patients with non-squamous NSCLC who received a long-term period of maintenance bevacizumab.
Patients and methods
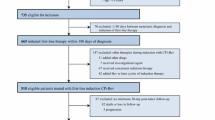
This retrospective study included 104 patients who had already reached a progression-free survival (PFS) of at least 9 months.
Results
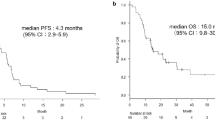
Median overall survival and PFS were 30.7 and 15.1 months, respectively. The overall response rate was 83 %. Weight loss ≤5 %, ECOG PS = 0, or low number of metastatic sites seem to be predictive factors of good evolution. The incidence of bevacizumab-related adverse events appeared to be similar as the previous studies.
Conclusion
Our findings show that there is a long-term survivor group whom the administration of bevacizumab resulted in a relevant prolongation of response without new safety signals. Due to the population heterogeneity, it was not possible to identify the standardised predictive factors.



Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Malvezzi M, Bertuccio P, Levi F, La VC, Negri E. European cancer mortality predictions for the year 2012. Ann Oncol. 2012;23(4):1044–52.
Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC lung cancer staging project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–14.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin XQ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4(9):1094–103.
Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400.
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–91.
Niho S, Kunitoh H, Nokihara H, Horai T, Ichinose Y, Hida T, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76(3):362–7.
Reck M, von PJ, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34.
Reck M, von PJ, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 2010;21(9):1804–9.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Zhou C, Wu YL, Chen G, Liu X, Zhu Y, Lu S, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–204.
Crino L, Dansin E, Garrido P, Griesinger F, Laskin J, Pavlakis N, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol. 2010;11(8):733–40.
Lopez-Chavez A, Young T, Fages S, Leon L, Schiller JH, Dowlati A, et al. Bevacizumab maintenance in patients with advanced non-small-cell lung cancer, clinical patterns, and outcomes in the eastern cooperative oncology group 4599 study: results of an exploratory analysis. J Thorac Oncol. 2012;7(11):1707–12.
Nadler E, Yu E, Ravelo A, Sing A, Forsyth M, Gruschkus S. Bevacizumab treatment to progression after chemotherapy: outcomes from a U.S. community practice network. Oncologist. 2011;16(4):486–96.
Soria JC, Mauguen A, Reck M, Sandler AB, Saijo N, Johnson DH, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(1):20–30.
Berman RS, Portera CA Jr, Ellis LM. Biology of liver metastases. Cancer Treat Res. 2001;109:183–206.
Nathoo N, Chahlavi A, Barnett GH, Toms SA. Pathobiology of brain metastases. J Clin Pathol. 2005;58(3):237–42.
Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, et al. Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol. 2012;123(2):205–22.
Bar J, Goss GD. Tumor vasculature as a therapeutic target in non-small cell lung cancer. J Thorac Oncol. 2012;7(3):609–20.
Folkman J, Ryeom S. Is oncogene addiction angiogenesis-dependent? Cold Spring Harb Symp Quant Biol. 2005;70:389–97.
Barlesi F, Scherpereel A, Rittmeyer A, Pazzola A, Ferrer TN, Kim JH, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31(24):3004–11.
Johnson BE, Kabbinavar F, Fehrenbacher L, Hainsworth J, Kasubhai S, Kressel B, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2013;31(31):3926–34.
Patel JD, Socinski MA, Garon EB, Reynolds CH, Spigel DR, Olsen MR, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(34):4349–57.
Dansin E, Cinieri S, Garrido P, Griesinger F, Isla D, Koehler M, et al. MO19390 (SAiL): bleeding events in a phase IV study of first-line bevacizumab with chemotherapy in patients with advanced non-squamous NSCLC. Lung Cancer. 2012;76(3):373–9.
Kosty MP, Brahmer JR, Jahanzeb M, Kumar P, Robles R, Wozniak AJ, et al. Use of bevacizumab (BV) after induction therapy is associated with survival benefit in patients (pts) with non-small cell lung cancer (NSCLC) in the ARIES observational cohort study (OCS). Eur J Cancer. 2011;47(Suppl. 1):598 (abstr. 9020).
Mok T, Gorbunova V, Juhasz E, Szima B, Burdaeva O, Orlov S, et al. A correlative biomarker analysis of the combination of bevacizumab and carboplatin-based chemotherapy for advanced nonsquamous non-small-cell lung cancer: results of the phase II randomized ABIGAIL study (BO21015). J Thorac Oncol. 2014;9(6):848–55.
Massuti B, Jantus-Lewintre E, Gonzalez Arriba JL, Rodriguez Abreu D, Juan O, Domine M et al (2014) ANGIOMET: analysis of the correlations between angiogenic markers and outcome in patients (p) with advanced nonsquamous NSCLC (NS-NSCLC) treated with carboplatin, paclitaxel, and bevacizumab (CPB). J Clin Oncol 32, 2014 (suppl; abstr e19014).
Acknowledgments
Medical writing support was provided by Ana López-Ballesteros and Antonio Torres at Dynamic S.L., during the preparation of this paper. This work was supported by Roche Farma, S.A., Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical standards
This study was conducted in accordance with the Declaration of Helsinki, all its amendments, and national regulations. The study was approved by the Clinical Research Ethic Committee at Hospital Universitario La Paz (Madrid, Spain) and all patients gave their informed consent before study inclusion.
Conflict of interest
Dr. de Castro provided Scientific advice to Roche Farma S.A; Dr. Massuti participated as an Advisor for Roche Farma S.A.; Dr. Rolfo received a research grant from Sanofi Aventis, he was also a Speaker Bureau for Novartis, and received consultancy fees from Mylan Pharmaceuticals and Oncopass; Dr. Paz-Ares provided Scientific advice to Roche Farma S.A.; and JV Cardona was an employee for Roche Farma S.A. None of the other authors has declared any conflict of interest.
Rights and permissions
About this article
Cite this article
De Castro, J., González-Larriba, J.L., Vázquez, S. et al. Long-term survival in advanced non-squamous NSCLC patients treated with first-line bevacizumab-based therapy. Clin Transl Oncol 19, 219–226 (2017). https://doi.org/10.1007/s12094-016-1527-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1527-8