Abstract
Purpose
The aim of this phase II study was to evaluate the activity and safety of the combination of cisplatin and vinorelbine with thoracic radiotherapy in unresectable locally advanced stage III non-small cell lung cancer (NSCLC). The primary endpoint was the objective response rate (ORR). Secondary objectives included toxicity profile, progression-free survival (PFS), and overall survival (OS).
Materials and methods
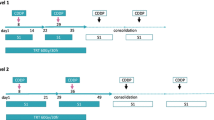
A total of 48 NSCLC patients were enrolled (median age 60 years, 52% stage IIIA and 48% stage IIIB, 52% adenocarcinoma). Patients received three cycles of chemotherapy every 21 days [intravenous cisplatin 80 mg/m2 and intravenous vinorelbine 25 mg/m2 on day 1 and oral vinorelbine on day 8 (60 mg/m2)] concurrent with radiotherapy (66 Gy, administered at 1.8 Gy per day, five consecutive days per week).
Results
ORR was 79.2% (72.9% showing partial response and 6.3% showing complete response). With a median follow-up of 20.7 months, median PFS was 12 months and median OS was 36 months. Grade 3/4 toxicities were: neutropenia (14.5%), anaemia (6.2%), vomiting (2%), and oesophagitis (4.2%). No toxic deaths were reported.
Conclusion
This combined regimen shows efficacy and a manageable safety profile. PFS and OS outcomes are encouraging and warrant further research.


Similar content being viewed by others
References
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):1–21. https://doi.org/10.1093/annonc/mdx222.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. https://doi.org/10.3322/caac.21442.
Tabchi S, Kassouf E, Rassy EE, Kourie HR, Martin J, Campeau MP, et al. Management of stage III non-small cell lung cancer. Semin Oncol. 2017;44(3):163–77. https://doi.org/10.1053/j.seminoncol.2017.10.009.
Auperin A, Le Pechoux C, Pignon JP, Koning C, Jeremic B, Clamon G, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17(3):473–83. https://doi.org/10.1093/annonc/mdj117.
Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90. https://doi.org/10.1200/jco.2009.26.2543.
Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103(19):1452–60. https://doi.org/10.1093/jnci/djr325.
Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17(9):2692–9. https://doi.org/10.1200/jco.1999.17.9.2692.
Steuer CE, Behera M, Ernani V, Higgins KA, Saba NF, Shin DM, et al. Comparison of concurrent use of thoracic radiation with either carboplatin-paclitaxel or cisplatin-etoposide for patients with stage III non-small-cell lung cancer: a systematic review. JAMA Oncol. 2017;3(8):1120–9. https://doi.org/10.1001/jamaoncol.2016.4280.
Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, et al. Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer (Amsterdam, Netherlands). 2004;46(1):87–98. https://doi.org/10.1016/j.lungcan.2004.03.004.
O’Rourke N, Roque IFM, Farre Bernado N, Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010;6:140. https://doi.org/10.1002/14651858.cd002140.pub3.
Lena MD, Ramlau R, Hansen O, Lorusso V, Wagner L, Barni S, et al. Phase II trial of oral vinorelbine in combination with cisplatin followed by consolidation therapy with oral vinorelbine in advanced NSCLC. Lung Cancer (Amsterdam, Netherlands). 2005;48(1):129–35. https://doi.org/10.1016/j.lungcan.2004.10.006.
Liang J, Bi N, Wu S, Chen M, Lv C, Zhao L, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol. 2017;28(4):777–83. https://doi.org/10.1093/annonc/mdx009.
Senan S, Brade A, Wang LH, Vansteenkiste J, Dakhil S, Biesma B, et al. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34(9):953–62. https://doi.org/10.1200/jco.2015.64.8824.
Vokes EE, Herndon JE 2nd, Crawford J, Leopold KA, Perry MC, Miller AA, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20(20):4191–8. https://doi.org/10.1200/jco.2002.03.054.
Marty M, Fumoleau P, Adenis A, Rousseau Y, Merrouche Y, Robinet G, et al. Oral vinorelbine pharmacokinetics and absolute bioavailability study in patients with solid tumors. Ann Oncol. 2001;12(11):1643–9.
Jassem J, Kosmidis P, Ramlau R, Zarogoulidis K, Novakova L, Breton J, et al. Oral vinorelbine in combination with cisplatin: a novel active regimen in advanced non-small-cell lung cancer. Ann Oncol. 2003;14(11):1634–9.
Jassem J, Ramlau R, Karnicka-Mlodkowska H, Krawczyk K, Krzakowski M, Zatloukal P, et al. A multicenter randomized phase II study of oral vs. intravenous vinorelbine in advanced non-small-cell lung cancer patients. Ann Oncol. 2001;12(10):1375–81.
Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK, Lee KC, et al. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33(24):2660–6. https://doi.org/10.1200/jco.2014.60.0130.
Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–99. https://doi.org/10.1016/s1470-2045(14)71207-0.
Krzakowski M, Provencio M, Utracka-Hutka B, Villa E, Codes M, Kuten A, et al. Oral vinorelbine and cisplatin as induction chemotherapy and concomitant chemo-radiotherapy in stage III non-small cell lung cancer: final results of an international phase II trial. J Thorac Oncol. 2008;3(9):994–1002. https://doi.org/10.1097/JTO.0b013e31818396cb.
Juan O, Sanchez-Hernandez A, Vazquez S, Casal J, Firvida JL, Aparisi F, et al. Full-dose cisplatin and oral vinorelbine concomitant with radiotherapy in unresectable stage III non-small cell lung cancer: a multi-center phase II study. Anticancer Res. 2014;34(4):1959–66.
Flentje M, Huber RM, Engel-Riedel W, Andreas S, Kollmeier J, Staar S, et al. GILT–A randomised phase III study of oral vinorelbine and cisplatin with concomitant radiotherapy followed by either consolidation therapy with oral vinorelbine and cisplatin or best supportive care alone in stage III non-small cell lung cancer. Strahlenther Onkol. 2016;192(4):216–22. https://doi.org/10.1007/s00066-016-0941-8.
Singhal N, Mislang A, Karapetis CS, Stephens S, Borg M, Woodman RJ, et al. Oral vinorelbine and cisplatin with concomitant radiotherapy in stage III non-small-cell lung cancer: an open-label phase II multicentre trial (COVeRT study). Anticancer Drugs. 2015;26(10):1083–8. https://doi.org/10.1097/cad.0000000000000291.
Sobin LHGM, Wittekind CH. International union against cancer (UICC) TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell; 2009.
Acknowledgements
Medical writing support was provided by Dr. Almudena Fuster-Matanzo of Medical Statistics Consulting S.L. (Valencia).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors had no conflict of interest in this study.
Ethical approval
The protocol of this trial was approved by the ethics committee of each participating hospital and met the ethical principles stated in the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Costa Rivas, M., Huidobro Vence, G., Fírvida Pérez, J.L. et al. Concurrent chemoradiation for locally advanced stage III non-small cell lung cancer with cisplatin, vinorelbine, and thoracic radiotherapy: a phase II study from the Galician Lung Cancer Group. Clin Transl Oncol 20, 1467–1473 (2018). https://doi.org/10.1007/s12094-018-1880-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1880-x