Abstract
Purpose
To determine the current management of oral and intravenous chemotherapy (OC and IVC) in outpatient clinics of Oncology Departments in Spain to detect opportunities for improvement.
Materials and methods
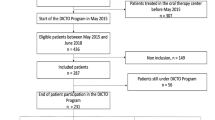
The Spanish Society of Medical Oncology designed a questionnaire specifically for Heads of Oncology Department: 142 were invited and 52 responded.
Results
In most centers, the waiting time (69.7%) and time at the outpatient clinic (84.8%) was shorter for patients receiving OC compared to those receiving IVC. The time spent at the outpatient clinic by the patients having OC was approximately 30 min (88.5%). In addition, the time expended by the oncologist with each patient was shorter when they were treated with OC in 21.2% of cases.
Conclusions
Patients’ waiting times and individual dedication of oncologists might be reduced by administering OC, and general management might be improved. This should be considered when planning therapies if OC is an option.


Similar content being viewed by others
References
Ramalho de Oliveira D, Brummel AR, Miller DB. Medication therapy management: 10 years of experience in a large integrated health care system. J Manag Care Pharm. 2010;16(3):185–95. https://doi.org/10.18553/jmcp.2010.16.3.185.
Jara C, Ayala F, Virizuela JA, Oncology Day Hospital Task F. The oncology day hospital in Spain: an updated analysis of Spanish Society of Medical Oncology (SEOM) looking forward. Clin Transl Oncol. 2017;19(3):269–72. https://doi.org/10.1007/s12094-016-1610-1.
El Miguel Servet necesita un nuevo hospital de día oncológico. Cadena Ser. 2015. http://cadenaser.com/emisora/2015/10/02/radio_zaragoza/1443807602_118256.html. Accessed June 2018.
Fernandez M. Denuncian masificación en el hospital de Día oncológico de Carlos Haya. La Opinión de Málaga. 2015. http://www.laopiniondemalaga.es/malaga/2015/04/11/denuncian-masificacion-hospital-oncologico-carlos/757532.html. Accessed June 2018.
Rubiera A. El aumento de pacientes fuerza a mejorar el hospital de día oncológico de Cabueñes. La nueva España. 2016. http://www.lne.es/gijon/2016/08/19/aumento-pacientes-fuerza-mejorar-hospital/1972182.html. Accessed June 2018.
El CHN mejora la seguridad y agiliza los tratamientos oncológicos. Redacción médica. 2017. https://www.redaccionmedica.com/autonomias/navarra/el-chn-mejora-la-seguridad-y-agiliza-los-tratamientos-oncologicos-6209. Accessed June 2018.
Biganzoli L, Lichtman S, Michel JP, Papamichael D, Quoix E, Walko C, et al. Oral single-agent chemotherapy in older patients with solid tumours: a position paper from the International Society of Geriatric Oncology (SIOG). Eur J Cancer. 2015;51(17):2491–500. https://doi.org/10.1016/j.ejca.2015.08.005.
García-Gómez R. [Abstract]. In: Garrido P, de Castro J (eds) XV congreso de la SEOM; Madrid (Spain): SEOM. 2015.
Strada MR, Palumbo R, Bernardo A, Riccardi A, Teragni C, Poggi G, et al. Intravenous or oral vinorelbine plus capecitabine as first-line treatment in HER2- metastatic breast cancer: joint analysis of 2 consecutive prospective phase II trials. Clin Breast Cancer. 2012;12(1):30–9. https://doi.org/10.1016/j.clbc.2011.11.001.
Jensen LH, Osterlind K, Rytter C. Randomized cross-over study of patient preference for oral or intravenous vinorelbine in combination with carboplatin in the treatment of advanced NSCLC. Lung Cancer. 2008;62(1):85–91. https://doi.org/10.1016/j.lungcan.2008.02.009.
European Medicines Agency. 2018. Capecitabine. Summary of Product Characteristics 2012. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002568/WC500135446.pdf. Accessed June 2018.
European Medicines Agency. Summary on Vepesid and associated names for etoposide in capsules. 2017. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Vepesid/human_referral_000419.jsp&mid=WC0b01ac05805c516f. Accessed June 2018.
European Medicines Agency. List of nationally authorised medicinal products with active substance vinorelbine. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Periodic_safety_update_single_assessment/2017/12/WC500239742.pdf. Accessed June 2018.
Barni S, Freier B, Garau I, Mouysset JL, Sediva M, Zamagni C, et al. Burden of advanced breast cancer for patients and caregivers in Europe: comparison of two treatment forms of vinorelbine, oral and intravenous. Curr Med Res Opin. 2016;32(11):1807–12. https://doi.org/10.1080/03007995.2016.1211518.
James RD. Improving chemotherapy capacity by switching from IV to oral vinorelbine. Eur J Oncol Pharm. 2010;4(3):14–7.
Wong SF, Bounthavong M, Nguyen CP, Chen T. Outcome assessments and cost avoidance of an oral chemotherapy management clinic. J Natl Compr Canc Netw. 2016;14(3):279–85.
Chewning B, Wiederholt JB. Concordance in cancer medication management. Patient Educ Couns. 2003;50(1):75–8.
Strasser F, Sweeney C, Willey J, Benisch-Tolley S, Palmer JL, Bruera E. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27(6):481–91. https://doi.org/10.1016/j.jpainsymman.2003.10.011.
Fortner BV, Tauer K, Zhu L, Okon TA, Moore K, Templeton D, et al. Medical visits for chemotherapy and chemotherapy-induced neutropenia: a survey of the impact on patient time and activities. BMC Cancer. 2004;4:22. https://doi.org/10.1186/1471-2407-4-22.
Le Lay K, Myon E, Hill S, Riou-Franca L, Scott D, Sidhu M, et al. Comparative cost-minimisation of oral and intravenous chemotherapy for first-line treatment of non-small cell lung cancer in the UK NHS system. Eur J Health Econ. 2007;8(2):145–51. https://doi.org/10.1007/s10198-006-0034-1.
Acknowledgements
We thank the 52 hospitals that have contributed to this study with their filled questionnaires. Blanca Piedrafita of Medical Statistics Consulting S.L. provided editorial support.
Funding
This study was a project by the Spanish Society of Medical Oncology (SEOM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AS has received an advisor honorarium from Roche Pharma, Astra Zeneca, Pierre-Fabre, Amgen and Clovis. JDC has received an advisor honorarium from Roche Pharma, Pfizer, Pierre-Fabre and Boehriger-Mannheim. ML has received an advisor honorarium from Roche Phrama, Astra Zeneca, Pierre-Fabre, Janssen, Boehringer-Mannheim and BMS. RV has received an advisor honorarium from Roche Pharma, Amgen and Sanofi. JM and EM have nothing to disclose.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santaballa, A., De Castro, J., Maurel, J. et al. Optimization of oral chemotherapy in outpatient clinics in Spain: results from a survey of the Spanish Society of Medical Oncology (SEOM). Clin Transl Oncol 21, 534–538 (2019). https://doi.org/10.1007/s12094-018-1951-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1951-z