Abstract
Small-cell lung cancer (SCLC) accounts for 15% of lung cancers. Only one-third of patients are diagnosed at limited stage. The median survival remains to be around 15–20 months without significative changes in the strategies of treatment for many years. In stage I and IIA, the standard treatment is the surgery followed by adjuvant therapy with platinum–etoposide. In stage IIB–IIIC, the recommended treatment is early concurrent chemotherapy with platinum–etoposide plus thoracic radiotherapy followed by prophylactic cranial irradiation in patients without progression. However, in the extensive stage, significant advances have been observed adding immunotherapy to platinum–etoposide chemotherapy to obtain a significant increase in overall survival, constituting the new recommended standard of care. In the second-line treatment, topotecan remains as the standard treatment. Reinduction with platinum–etoposide is the recommended regimen in patients with sensitive relapse (≥ 3 months) and new drugs such as lurbinectedin and immunotherapy are new treatment options. New biomarkers and new clinical trials designed according to the new classification of SCLC subtypes defined by distinct gene expression profiles are necessary.
Similar content being viewed by others
Introduction
Small-cell lung cancer (SCLC) is the most aggressive subtype of lung cancer, which is strongly associated with cigarette smoking. SCLC comprises about 15% of all lung cancer cases. A decreasing trend in the incidence has recently been observed, but is still increasing in women, what might reflect changes in smoking habit [1].
SCLC is characterised by a rapid doubling time, a high growth fraction, an early development of widespread metastases, paraneoplastic endocrinopathies and sensitivity to chemotherapy and radiation. Untreated SCLC is rapidly fatal within 2–4 months. According to the classification system developed by the veterans’ administration lung study group (VALSG), more than two-thirds of SCLC patients are diagnosed with extensive stage (ES) and the remaining patients are diagnosed with limited stage (LS).
In LS stage patients, 80–90% achieve a partial or complete response using a combination of chemotherapy plus radiation with a median overall survival (mOS) of 15–20 months. Not more than 10–20% of patients survive beyond 5 years. In ES, mOS is 8–13 months, with a 5-year survival rate < 2% [2].
Although favourable response rates (ORR) have been achieved using combination chemotherapy, particularly platinum–etoposide as the most widely used regimen, only a small proportion of patients survive after 5 years. Most patients relapse within 1 year of starting first-line treatment and the reasons for the intrinsic or acquired resistance to chemotherapy are still unclear.
Methodology
For the elaboration of this guideline, we have carried out an exhaustive review of the most relevant published studies to date. The Infectious Diseases Society of America grading system was used to assign levels of evidence and grades of recommendation [3].
Pathological diagnosis
The pathological diagnosis of SCLC should be made using the World Health Organization (WHO) classification [4]. SCLC is a type of neuroendocrine tumour (NET) of the lung that consists of small cells with scant cytoplasm, poorly defined cell borders, fine granular nuclear chromatin, and absent or inconspicuous nucleoli. The cells are round-, oval-, or spindle-shaped, and the nuclear moulding is prominent. The classic and distinctive histology on haematoxylin and eosin (H&E) may be sufficient for identifying SCLC in good-quality histologic or cytologic samples, but immunohistochemistry (IHC) may be required for confirmation, including synaptophysin, chromogranin, and NCAM/CD56. The mitotic count is high (at least 10 mitoses/2 mm2, but averaging over 60 mitoses/2 mm2) and the proliferative index as evaluated by Ki-67 antigen IHC is > 50%, approaching 100% in most cases.
Initial evaluation and staging
Initial assessment must include medical and smoking histories, physical examination, complete blood count, biochemistry including liver enzymes, sodium, potassium, calcium, glucose, lactate dehydrogenase levels and renal function tests, and, in the case of thoracic radiation, lung function tests.
Full staging includes computed tomography (CT) scan (with intravenous contrast) of the chest/abdomen; and brain imaging using magnetic resonance imaging (MRI) (preferred) or CT scan (with intravenous contrast). If LS is suspected, a 2-fluor-2-deoxy-d-glucose positron-emission-tomography (FDG-PET) CT could be performed to assess distant metastases. PET scans can increase staging accuracy in patients with SCLC, because SCLC is a highly metabolic disease. Approximately 19% of patients who undergo PET/CT are upstaged from LS to ES disease. PET/CT is recommended in LS patients. In patients with a solitary metastasis, its pathological confirmation is recommended to clarify the stage (III, C).
The 8th edition of the TNM staging system according to the AJCC should be used to define better prognosis and personalised treatment options (I, A) [5]. The VALSG classification could be used in clinical practice [6]. LS is defined as stage I–III (T any, N any, M0) that can be treated with definitive chemoradiation therapy. ES is defined as stage IV (T any, N any, M1a/b/c) or T3–4 due to multiple lung nodules that are too extensive or have tumour/nodal volume that is too large to be encompassed in a tolerable radiation plan (Table 1).
Limited stage
The mOS for patients with LS is 15–20 months and 5-year survival rate is 10–20%.
Stage I–IIA (T1–T2, N0, M0)
Stage I–IIA (T1–2, N0, M0) represent less than 5% of SCLC. In these patients, a mediastinal staging is necessary that should be performed by either surgery (mediastinoscopy, mediastinotomy or video-assisted thoracoscopy) or endobronchial or oesophageal ultrasound-guided biopsy (II, A). The lobectomy should be the preferred surgical procedure with a systematic lymph-node dissection (II, A). Adjuvant systemic therapy should be always considered (II, A). For patients with surgical contraindication or refusing surgery, stereotactic body radiation therapy (SBRT) may be a useful treatment (III, B). Sequential chemotherapy after SBRT yielded a mOS of 31.4 vs 14.3 months compared to SBRT alone (p = 0.02) [7]. Four cycles of cisplatin–etoposide are the recommended systemic adjuvant therapy (Table 2) in this subgroup of patients (II, A). Adjuvant radiotherapy, administered sequentially or concomitantly to chemotherapy, should be recommended when N1 or N2 disease has been diagnosed after surgery (II, A). There is no clear evidence of prophylaxis cranial irradiation (PCI) recommendation in surgically resected early stage patients (T1–2, N0, M0), since data from this subgroup of patients have lower risk to develop brain metastases [8]. For this reason, we do not recommend its use in T1–2, N0, MO, patients.
Stage IIB–IIIC (T3–4, N0, M0; T 1–4, N1–3, M0)
The standard treatment in these patients is concurrent chemotherapy and thoracic radiotherapy (CTRT) (IA).
Chemotherapy should be administered up to a maximum of 4–6 cycles. Cisplatin plus etoposide is the recommended chemotherapy regimen to combine with thoracic radiotherapy (I, A) [9, 10]. There is less evidence for the use of carboplatin, although a meta-analysis including 663 patients (32% with LS) demonstrated no significant differences when comparing cisplatin vs carboplatin, with an ORR of 67% vs 66%, median progression free survival (mPFS) of 5.5 vs 5.3 months, and mOS of 9.6 vs 9.4 months. However, carboplatin should be reserved only when cisplatin is contraindicated (Table 2) [11]. There is no recommendation for the use of prophylactic myeloid growth factor to avoid myelosuppression (I, A) in this setting.
The use of thoracic radiotherapy for LS-SCLC has demonstrated an improvement of 25–30% of reduction of local recurrence and a 5–7% improvement in 2-year survival rate vs chemotherapy alone. However, the optimal dose and schedule of thoracic RT has not been definitively established and different factors such as, performance status (PS), pulmonary function, target volume, comorbidities, and centre resources may underlie their election. Early concurrent CTRT has demonstrated better local control and survival results when compared to sequential treatment administration [12,13,14] at the cost of a higher rate of oesophageal toxicity. With respect to fractionation, both once-daily and twice-daily radiotherapy with cisplatin and etoposide have been evaluated in LS-SCLC. The ECOG–RTOG trial demonstrated a survival advantage of the twice-daily approach with higher grade 3–4 toxicity [9]. The CONVERT trial demonstrated a similar efficacy result, when comparing both strategies, with higher grade 4 neutropenia events for patients receiving twice-daily radiotherapy [10]. For once-daily radiotherapy, the recommended schedule is 2.0 Gy/day up to 60–70 Gy, and for twice-daily radiotherapy, the recommended dose is 1.5 Gy twice a day, 30 fractions up to a total dose of 45 Gy. Radiotherapy administered concomitantly to chemotherapy is the standard treatment that demonstrated superior results when compared to sequential treatment (I, A). Moreover, a shorter time elapsed from the initiation of any therapy to the end of radiotherapy has associated improved survival. Thus, radiotherapy should be started as early as with the first or second course of chemotherapy (I, A) [12]. The radiotherapy target field should be defined according to the PET/CT scan performed prior to treatment initiation and should include the involved node regions, as well as the primary tumour [15].
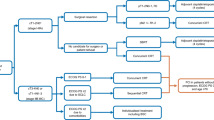
PCI is well established to decrease the incidence of brain metastases after systemic control and to prevent morbidity and mortality usually associated with developing brain metastases. A meta-analysis of seven randomised trials including 987 patients who achieved complete remission with systemic treatment and received PCI showed that the incidence of brain metastases was significantly lower with PCI (RR 0.46, 95% CI 0.38–0.57) and reported improvement of three years cumulative incidence of brain metastases (33% vs 59%). Furthermore, overall survival (OS) at 3 years was improved from 15.3% to 20.7% with PCI (RR 0.84, 95% CI 0.73–0.97) [16]. Moreover, a randomised trial of 720 patients with complete remission after CTRT demonstrated that 25 Gy in 10 fractions as PCI was effective, equal to higher doses, and less toxic [17]. Advance age and doses > 25 Gy have demonstrated a higher risk of chronic neuro-cognitive impairment [18]. PCI (25 Gy) should be administered after CTRT in patients without progression (I, A). Treatment algorithm for treatment of LS is shown in Fig. 1.
Treatment algorithm for limited stage SCLC. LN lymph node, CT chemotherapy (cisplatin/etoposide, alternative carboplatin/etoposide 4 cycles), RT radiotherapy, ECOG Eastern Cooperative Oncology Group, PS performance status,SCLC small-cell lung cancer, BSC best supportive care, PCI prophylactic cranial irradiation
Extensive stage
The mOS for patients with ES-SCLC is about 8–13 months. Patients treated with platinum compound relapse within 6 months and less than 2% survive beyond 2 years [2] . Algoritm of treatment of ES SCLC is described in Fig. 2.
Treatment algorithm for extensive stage and second or successive lines. ECOG Eastern Cooperative Oncology Group, PS performance status, PCI prophylactic cranial irradiation, MRI magnetic resonance imaging, RT radiotherapy, CT chemotherapy, G-CSF granulocyte colony-stimulating factor, BSC best supportive care
First-line treatment
The first-line treatment of ES-SCLC has changed recently with the use of the combination of chemotherapy and immunotherapy. Chemotherapy alone remains as an effective option in patients with contraindication for immunotherapy. Schedule regimens for systemic treatment in first-line treatment are described in Table 2.
Combined chemotherapy and immunotherapy
The combination of chemotherapy and immunotherapy is currently considered the standard first-line treatment of ES-SCLC.
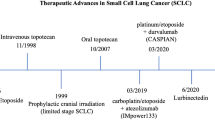
In the randomised phase III IMpower 133 trial [19], 403 patients with ES-SCLC, were assigned to receive four cycles of carboplatin and etoposide with atezolizumab or placebo, followed by maintenance with either atezolizumab or placebo. This trial showed a significant benefit for atezolizumab in mOS, 12.3 vs 10.3 months, HR 0.70 (0.54–0.91, p = 0.0069). An updated OS was reported in ESMO 2019 after a median follow-up of 22.9 months and demonstrated an increase in the overall survival at 18 months by 13% in the atezolizumab arm (34% vs 21%). The incidence of toxicity of any grade was similar between the two groups. Blood tumour mutation burden and PD-L1 did not show to be predictive biomarkers [20]. On March 18, 2019, the food and drug administration (FDA) approved atezolizumab in combination with carboplatin and etoposide, for the first-line treatment of adult patients with ES-SCLC, followed by the European medicines agency (EMA) on September 6, 2019 (I, A).
The phase III CASPIAN trial evaluated durvalumab plus etoposide and cis/carboplatin in first-line ES-SCLC demonstrating a statistically significant improvement in mOS: 13.0 vs 10.3 months, HR 0.73 (95% CI 0.59–0.91; p = 0.0047) for durvalumab. The combination of durvalumab with either cisplatin or carboplatin–etoposide could stand as a new treatment option in first-line for ES-SCLC (I, A) [21].
By contrast, ipilimumab (cytotoxic T-lymphocyte associated protein 4 antibody), when added to chemotherapy, improves PFS, but not OS in treatment-naïve ES-SCLC [22].
Chemotherapy
Carboplatin or cisplatin plus etoposide
Cisplatin plus etoposide has been the standard treatment for patients with ES-SCLC (I, A) for decades. A meta-analysis that included individual patient data from four trials found no statistically significant difference in OS between carbo or cisplatin-based combinations. Carboplatin–etoposide could be an alternative regimen in patients with contraindications for cisplatin (I, A) [11].
Alternative regimens to platinum–etoposide
Camptothecin-based regimens
Several clinical trials have studied the substitution of a camptothecin analogue for etoposide in combination with a platinum compound in patients with ES-SCLC. In the Japanese Cooperative Oncology Group trial (JCOG 9511), patients treated with irinotecan plus cisplatin had a significantly longer mOS compared with etoposide–cisplatin (12.8 vs 9.4 months) [23]; however, other larger trials including cisplatin or carboplatin conducted outside Japan failed to confirm this observation. A recent meta-analysis reported better OS with platinum–irinotecan, but equal ORR and PFS than platinum–etoposide with less haematologic and greater gastrointestinal toxicity with irinotecan [24].
Epirubicin plus cisplatin
In a randomised phase III trial, the combination of epirubicin and cisplatin was similar to cisplatin and etoposide in ORR, mPFS (7.6 months in both arms) and mOS (10.9 versus 10.1 months) [25]. The combination of epirubicin plus cisplatin could be a reasonable alternative regimen in this setting.
Other combinations testing topotecan–cisplatin or amrubicin–cisplatin have not demonstrated an advantage over the standard platinum–etoposide combination. The addition of a third drug to platinum–etoposide regimen failed to show a significant difference in OS.
Alternative regimens are cisplatin–irinotecan, carboplatin–irinotecan, cisplatin, and epirubicin (II, B).
Duration of treatment
The optimal duration of chemotherapy for patients with ES-SCLC is not well defined; in general, 4–6 cycles are recommended. A meta-analysis reported that maintenance chemotherapy did not prolong OS [26]. No other target drugs have demonstrated benefit in OS as maintenance.
Radiotherapy in ES-SCLC
Prophylactic cranial irradiation (PCI) in ES-SCLC
Prevention of cranial progression is a major concern in LS and ES-SCLC. A meta-analysis [16] suggested a benefit with PCI in responding patients, but most patients had LS-SCLC. In ES-SCLC who has responded to systemic therapy, PCI decreases the development of brain metastases. Conflicting results were obtained in two-phase III trials regarding improvement of mOS with PCI after response to systemic chemotherapy. The EORTC trial showed an OS improvement with PCI [27]. A Japanese phase III trial showed that PCI did not improve OS in patients without brain metastases at baseline MRI compared with follow-up with MRI and treatment after detection of asymptomatic brain metastases [28]. PCI may be considered in good PS responding patients (I, B). Follow-up with brain MRI is recommended for all patients regardless of the administration of PCI. Depending on individual patient factors, close MRI surveillance should be an appropriate option for ES-SCLC patients who achieve a response to initial systemic therapy.
Patients should be informed of the potential adverse effects from PCI. PCI is not recommended for patients at high risk of neurological sequelae (PS 3–4 or neuro-cognitive impairment) and should be used with caution in elderly patients.
Preferred schedule is 25 Gy in ten daily fractions.
Treatment of brain metastases
In symptomatic brain metastases, whole-brain radiotherapy (WBRT) before systemic therapy should be administered (II, A). In patients with a small number of metastases, SBRT should be an optional treatment. In asymptomatic brain metastasis, systemic therapy should be administered as the first treatment with cranial MRI or CT with contrast evaluation during systemic therapy; WBRT may be administered after completion of systemic therapy or if brain metastases progress during systemic therapy. Preferred schedule for WBRT is 30 Gy in ten daily fractions. In patients with cranial progression after PCI: consider to repeat WBRT or stereotactic radiosurgery (preferably) if feasible.
Consider adding memantine during and after radiotherapy (PCI or WBRT).
Consolidative thoracic radiation therapy
As a result of two phase III trials [29, 30], consolidative thoracic radiotherapy should be considered in selected patients with ES-SCLC who have completed chemotherapy and achieved a complete or near complete response, especially in patients with good extrathoracic response (I, B).
Second and successive lines in ES-SCLC
Despite SCLC being very responsive to initial therapy, most of the patients relapse with a mOS of 5–7 months. It is very important for the distinction of > 3 months (chemo-sensitive disease) or within 3 months (chemo-resistant or refractory disease). All patients with relapsed SCLC should be assessed for clinical trials. Decision treatment should include PS, comorbidities, toxicity, and disease-free interval from prior therapy. When a patient relapses more than 3 months after the first-line therapy, reinduction of the original regimen with platinum etoposide is recommended (II, B) [31]. If relapse occurs, 3 months or less must be considered administering single-agent therapy with IV or oral topotecan (I, B). An alternative is the combination CAV: cyclophosphamide–doxorubicin–vincristine (II, B). Other agents commonly used based on phase 2 trials are irinotecan, taxanes, gemcitabine, vinorelbine, or temozolomide [32]. Only around 20% of SCLC patients will receive the third-line therapy with modest results.
A novel cytotoxic drug is lurbinectedin, a transcription inhibitor that binds to the DNA minor groove and inhibits RNA polymerase II; is active as a single agent in second-line SCLC in a phase 2 trial for both sensitive and resistant disease (ORR 35.2%) [33], a phase 3 study in combination with doxorubicin vs chemotherapy has completed the recruitment, pending of final results (ATLANTIS trial).
Several targeted therapies have been assessed without still satisfactory results. Rovalpituzumab is an antibody–drug conjugate directed against DLL3 (Notch signalling) with positive results in an initial trial [34], but negative in a phase 3 study against topotecan (TAHOE trial). Other studies with DLL3 inhibitors are ongoing. New drugs targeting other new pathways are in development, including DNA damage and repair (e.g. PARP inhibitors), epigenetics, and cell cycle.
Immunotherapy with immune checkpoint inhibitors has demonstrated modest activity in relapsed SCLC patients (nivolumab +/− ipilimumab, pembrolizumab, atezolizumab, and durvalumab + tremelimumab) without clear predictive biomarker identification; and the only phase 3 study carried out comparing nivolumab vs standard chemotherapy (CheckMate 331) was negative [35]. New immunotherapy drugs and combinations remain promising. Patients whose progress while on immunotherapy as part of first-line therapy should not be treated with other immune checkpoint inhibitors (see Fig. 2).
Definitely, predictive biomarker-driven therapies are needed with the aim to improve the current still poor outcomes in relapsed SCLC. New classification of SCLC subtypes defined by distinct gene expression profiles could help us to design new clinical trials [36]. Schedules regimens for second and successive lines are described in Table 2.
Treatment of fragile and elderly patients
The incidence of SCLC increases with age; approximately one-third of patients are 70 years of age or older. The key to treatment is to assess whether the expected benefits of treatment are superior to the risks. In general, good performance older adult patients should receive the same doses as younger patients.
In LS-SCLC, the concurrent CTRT with modern radiotherapy techniques could be a treatment option for fit, older patients. This approach yields equivalent mOS in older vs younger patients (29 vs 30 months; p = 0.38) [37].
In ES-SCLC, older patients with good PS are able to tolerate commonly used chemotherapy with adequate supportive care. Randomised trials have indicated that less-intensive treatment (e.g., single-agent) is inferior to combination chemotherapy in elderly patients with good PS (0–2). Dose attenuation and carboplatin-based regimens are preferred in risk patients, although there are associated with poor therapeutic outcomes [38]. Myelosuppression, fatigue, and lower organ reserves are found more frequently in elderly patients. The most important risk factor is the development of severe neutropenia; moreover the treatment, is older age (> 65 years). The use of colony-stimulating factors (G-CSF) in the elderly patients is recommended [39].
PCI should be used with caution in elderly patients; an increased risk for cognitive decline after PCI is found in patients (≥ 60 years) [40].
There are no data that define the role of treatment in poor PS patients (PS3 or PS4) or extremes ages; it seems reasonable to offer treatment to this patient if their poor PS is due to SCLC rather than comorbidity.
Follow-up
The smoking cessation is the most important recommendation for patients with SCLC. The objective of the follow-up is to detect early relapse. There are not clinical trials evaluating the follow-up of SCLC. For LS, a CT scan is recommended every 3 months the first year, every 6 months years 2–5, and then annually (V, C). For ES every 2 months, the first year, every 3 months year 2 and 3, every 6 months year 4–5, and then annually (V, C).
Summary of recommendations is listed in Table 3.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
- De Castro Carpeño J, Cobo Dols M, Domine Gomez M, Ruiz Gracia P, Crama L, Garcia Campelo R. Survival outcomes in stage IV small cell lung cancer (IV-SCLC). Analysis from SEER database. ESMO immuno-oncology congress 2019 (abs 418)
Khan AR, Khan S, Zimmerman V, et al. Quality and strength of evidence of the infectious diseases Society of America Clinical Practice Guidelines. Clin Infect Dis. 2010;51(10):1147–56.
Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer; 2015.
Amin MB, Greene FL, Byrd DR, et al. American Joint Committee on Cancer (AJCC) cancer staging manual. 8th ed. New York: Springer; 2016. p. 1–1024.
Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;37:271–6.
Verma V, Simone CB 2nd, Allen PK, Lin SH. Outcomes of stereotactic body radiotherapy for T1–T2N0 small cell carcinoma according to addition of chemotherapy and prophylactic cranial irradiation: a multicenter analysis. Clin Lung Cancer. 2017;18:675–81.
Yang Y, Zhang D, Zhou X, et al. Prophylactic cranial irradiation in resected small cell lung cancer: a systematic review with meta-analysis. J Cancer. 2018;9:433–9.
Turrisi AT, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71.
Faivre-Finn C, Snee M, Ashcroft L, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–25.
Rossi A, Di Maio M, Chiodini P, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol. 2012;30:1692–8.
Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–60.
Fried DB, Morris DE, Poole C, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–45.
De Ruysscher D, Lueza B, Le Péchoux C, et al. Impact of thoracic radiotherapy timing in limited-stage small-cell lung cancer: usefulness of the individual patient data meta-analysis. Ann Oncol. 2016;27:1818–28.
Bogart JA, Herndon JE, Lyss AP, et al. 70 Gy thoracic radiotherapy is feasible concurrent with chemotherapy for limited-stage small-cell lung cancer: analysis of Cancer and Leukemia Group B study 39808. Int J Radiat Oncol Biol Phys. 2004;59:460–8.
Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission: Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–84.
Le Péchoux C, Dunant A, Senan S, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99–01, EORTC 22003-08004, RTOG 0212, and IFCT 99-01): a randomised clinical trial. Lancet Oncol. 2009;10:467–74.
Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:77–84.
Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379:2220–9.
Reck M, Liu SV, Mansfield AS, et al. IMpower 133: updated overall survival analysis of first-line atezolizumab + carboplatin + etoposide in extensive-stage SCLC. ESMO congress. Barcelona 2019. Ann Oncol 2019; 30 Suppl 5 (abstract 17360).
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–39.
Reck M, Luft A, Szczesna A, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34:3740–8.
Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85–91.
Liu ZL, Wang B, Liu JZ, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin in patients with previously untreated extensive-stage small cell lung cancer: a meta-analysis. J Cancer Res Ther 2018;14 (Suppl):S1076–83.
Artal-Cortés A, Gomez-Codina J, Gonzalez-Larriba JL, et al. Prospective randomized phase III trial of etoposide/cisplatin versus high-dose epirubicin/cisplatin in small-cell lung cancer. Clin Lung Cancer. 2004;6:175–83.
Zhou H, Zeng C, Wei Y, Zhou J, Yao W. Duration of chemotherapy for small cell lung cancer: a meta-analysis. PLoS One. 2013;8:e73805.
Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–72.
Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, pase 3 trial. Lancet Oncol. 2017;18:663–71.
Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42.
Palma DA, Warner A, Louie AV, et al. Thoracic radiotherapy for extensive stage small-cell lung cancer: a meta-analysis. Clin Lung Cancer. 2016;17:239–44.
Baize N, Monnet I, Greillier L, et al. Carboplatin-etoposide versus topotecan as second-line treatment for sensitive relapsed small-cell lung cancer: phase 3 trial. J Thorac Oncol. 2019;14(Suppl):S246.
Gong J, Salgia R. Managing patients with relapsed small-cell lung cancer. J Oncol Pract. 2018;14:359–66.
Paz-Ares L, Trigo JM, Besse B, et al. Efficacy and safety profile of lurbinectedin in second-line SCLC patients: Results from a phase II single-agent trial. J Clin Oncol 2019; 37 (suppl) (abstr 8605).
Rudin CM, Pietanza MC, Bauer TM, et al. Rovalpituzumabtesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open label, phase 1 study. Lancet Oncol. 2017;18:42–51.
Reck M, Vicente D, Ciuleanu T, et al. Efficacy and safety of nivolumab monotherapy versus chemotherapy in recurrent small cell lung cancer: results from CheckMate 331. Ann Oncol 2018;29(Supp 10) (abs LBA5).
Rudin CM, Poirier JT, Byers LA, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–97.
Christodoulou M, Blackhall F, Mistry H, et al. Compliance and outcome of elderly patients treated in the concurrent once-daily versus twice-daily radiotherapy (CONVERT) trial. J Thorac Oncol. 2019;14:63–71.
Girling DJ. Comparison of oral etoposide and standard intravenous multidrug chemotherapy for small-cell lung cancer: a stopped multicentre randomised trial: Medical Research Council Lung Cancer Working Party. Lancet. 1996;348:563–6.
Feliu J, Heredia-Soto V, Gironés R, et al. Management of the toxicity of chemotherapy and targeted therapies in elderly cancer patients. Clin Transl Oncol. 2019;25:1–11.
Damhuis RAM, Senan S, Belderbos JS. Usage of prophylactic cranial irradiation in elderly patients with small-cell lung cancer. Clin Lung Cancer. 2018;19:263–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MD reports lecture and advisory board from AstraZeneca, BMS and Boehringer Ingelheim, outside the submitted work. TM has nothing to disclose. DI reports personal financial interests: Consulation Honoraria from AbbVie, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, F. Hoffmann-La Roche, MSD, Pierre Fabre, Pfizer, and Takeda; Speaker Honoraria from AstraZeneca, Bayer, BMS, Boehringer Ingelheim, F. Hoffmann-La Roche, MSD, Pierre Fabre and Pfizer. Institutional financial interests: Clinical Trials from AbbVie, AstraZeneca, Boehringer Ingelheim, BMS, F. Hoffmann-La Roche, Janssen, Merck, MSD, Novartis; Research Grant from BMS, F. Hoffmann-La Roche. Non-financial interests: Leadership Role from SEOM (Spanish Society of Medical Oncology): Scientific Assistant. Other: Executive Board Member of the Commission for the Approval of New Drugs, Regional Health Care Department, Spain. JLM reports honoraria from Roche, outside the submitted work. IS reports personal fees from Roche, MSD, BMS, AstraZeneca, Boehringer Ingelheim, Pfizer and Novartis, and non-financial support from Novartis, outside the submitted work. MP reports honoraria for speaking and advisory board from BMS, AstraZeneca, Takeda, Roche and Amgen, outside the submitted work. MEO has nothing to disclose. SP has nothing to disclose. AB has nothing to disclose. MC reports lecture and advisory board from AstraZeneca, BMS, Boehringer Ingelheim, outside the submitted work.
Ethical approval
The current study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dómine, M., Moran, T., Isla, D. et al. SEOM clinical guidelines for the treatment of small-cell lung cancer (SCLC) (2019). Clin Transl Oncol 22, 245–255 (2020). https://doi.org/10.1007/s12094-020-02295-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02295-w