Abstract
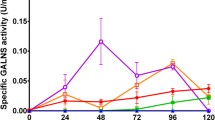
The sulfatase family involves a group of enzymes with a large degree of similarity. Until now, sixteen human sulfatases have been identified, most of them found in lysosomes. Human deficiency of sulfatases generates various genetic disorders characterized by abnormal accumulation of sulfated intermediate compounds. Mucopolysaccharidosis type II is characterized by the deficiency of iduronate 2-sulfate sulfatase (IDS), causing the lysosomal accumulation of heparan and dermatan sulfates. Currently, there are several cases of genetic diseases treated with enzyme replacement therapy, which have generated a great interest in the development of systems for recombinant protein expression. In this work we expressed the human recombinant IDS-Like enzyme (hrIDS-Like) in Escherichia coli DH5α. The enzyme concentration revealed by ELISA varied from 78.13 to 94.35 ng/ml and the specific activity varied from 34.20 to 25.97 nmol/h/mg. Western blotting done after affinity chromatography purification showed a single band of approximately 40 kDa, which was recognized by an IgY polyclonal antibody that was developed against the specific peptide of the native protein. Our 100 ml-shake-flask assays allowed us to improve the enzyme activity seven fold, compared to the E. coli JM109/pUC13-hrIDS-Like system. Additionally, the results obtained in the present study were equal to those obtained with the Pichia pastoris GS1115/pPIC-9-hrIDS-Like system (3 L bioreactor scale). The system used in this work (E. coli DH5α/pGEX-3X-hrIDS-Like) emerges as a strategy for improving protein expression and purification, aimed at recombinant protein chemical characterization, future laboratory assays for enzyme replacement therapy, and as new evidence of active putative sulfatase production in E. coli.
Similar content being viewed by others
References
Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W.J., and Lipman, D. 1997. Gapped BLAST and PSI-LAST: A new generation of protein database search programs. Nucleic Acid Res. 25, 3389–3402.
Archer, I.M., Harper, P.S., and Wusteman, F.S. 1982. Multiple forms of Iduronate 2-sulphate sulphatase in human tissues and body fluids. Biochem. Biophys. Acta. 708, 134–140.
Bader, R. and Leisinger, T. 1994. Isolation and characterization of the Methylophilus sp. strain DMll gene encoding dichloromethane dehalogenase/glutathione S-transferase. J. Bacteriol. 176, 3466–3473.
Baneyx, F. and Mujacic, M. 2004. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22, 1399–1408.
Barrera, L.A. 1990. Enfermedades genéticas de origen metabólico. Pediatría 25, 64–68.
Barrera, L.A. 1993. Errores innatos del metabolismo. Acta Méd. Colomb. 18, 31–40.
Beaudet, A.L., Scriver, C.R., Sly, W.S., and Valle, D. 2001. The metabolic and molecular bases of inherited disease. McGraw-Hill.
Benjdia, A., Deho, G., Rabot, S., and Berteau, O. 2007. First evidences for a third sulfatase maturation system in prokaryotes from E. coli aslB and ydeM deletion mutants. FEBS Lett. 581, 1009–1014.
Bielicki, J., Freeman, C., Clements, P.R., and Hopwood, J.J. 1990. Human liver iduronate 2-sulfatase. Purification, characterization and catalytic properties. Biochem. J. 271, 75–86.
Bielicki, J., Hopwood, J., Wilson, P.J., and Anson, D.S. 1993. Recombinant human iduronate -2-sulphatase: correction of mucopolysaccharidosis — type II fibroblasts and characterization of the purified enzyme. J. Biochem. 289, 241–246.
Bond, C.S., Clements, P.R., Ashby, S.J., Collyer, C.A., Harrop, S., Hopwood, J.J., and Guss, M. 1997. Structure of human lysosomal sulfatase. Struct. 5, 277–289.
Córdoba-Ruiz, H.A., Poutou-Piñales, R.A., Echeverri-Peña, O.Y., Algecira-Enciso, N.A., Landázuri, P., Sáenz, H., and Barrera-Avellaneda, L.A. 2009. Laboratory scale production of the human recombinant iduronate 2-sulfate sulfatase-Like from Pichia pastoris. Afr. J. Biotechnol. 8, 1786–1792.
Dakterzada, F., Mobarez, A.M., Roudkenar, M.H., and Forouzandeh, M. 2012. Production of pentameric cholera toxin B subunit in Escherichia coli. Avicenna J. Med. Biotechnol. 4, 89–94.
Di Natale, P. and Ronsisville, L. 1981. Identification and partial characterization of two enzymes forms of iduronate sulfatase from human placenta. Biochem. Biophys. Acta. 661, 106–111.
Dierks, T., Lecca, M.R., Schlotterhose, P., Schmidt, B., and von Figura, K. 1999. Sequence determinants directing conversion of cysteine to formylglycine in eukariotic sulfatases. Embo. J. 18, 2084–2091.
Dierks, T., Lecca, M.R., Schmidt, B., and von Figura, K. 1998a. Conversion of cysteine to formylglycine in eukaryotic sulfatases occurs by a common mechanism in the endoplasmic reticulum. FEBS Lett. 423, 61–65.
Dierks, T., Miech, C., Hummerjohann, J., Schmidt, B., Kertesz, M.A., and von Figura, K. 1998b. Posttranslational formation of formylglycine in prokaryotic sulfatases by modification of either cysteine or serine. J. Biol. Chem. 273, 25560–25564.
Dong, X., Stothard, P., Forsythe, I., and Wishart, D. 2004. Plas-Mapper: a web server for drawing and auto-annotating plasmid maps. Nucleic Acids Res. 32, W660–664.
Friedman, B., Vaddi, K., Preston, C., Mahon, E., Cataldo, J.R., and Macpherson, J.M. 1999. A comparison of pharmacological properties of carbohydrate remodeled recombinant and placental-derived β-glucocerebrosidase: Implications for clinical efficacy in treatment of Gaucher disease. Blood 93, 2807–2816.
Froissart, R., Millat, G., Mathiu, M., Bozon, D., and Maire, I. 1995. Processing of iduronate 2-sulfatase in human fibroblasts. Biochem. J. 309, 425–430.
García, A.R., DaCosta, J.M., Pan, J., Muenzer, J., and Lamsa, J.C. 2007. Preclinical dose ranging studies for enzyme replacement therapy with idursulfase in a knock-out mouse model of MPS II. Mol. Gen. Metabol. 91, 183–190.
Gasteiger, E., Hoogland, C., Gattiker, A., Duvaud, S., Wilkins, M.R., Appel, R.D., and Bairoch, A. 2005. Protein identification and analysis tools on the ExPASy server, pp. 571–660. Humana Press, New York, USA.
Guez, N. and Peitisch, M.C. 1997. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electroph. 18, 2714–2723.
Hilman, B.C. and Sorensen, R.U. 1994. Management options: SCIDS with adenosine deaminase deficiency. Ann. Aller. 72, 395–402.
Hopwood, J.J. and Ballabio, A. 2001. Multiple sulfatase deficiency and the nature of sulfatae family. In Scriver, C.R., Beaudet, A., Sly, W., and Valle, D. (eds.), pp. 3725–3732. The metabolic and molecular bases of inherited disease. Mc Graw-Hill, New York, N.Y., USA.
Invitrogen. 2001. Subcloning efficiency DH5α. Catalog no. 18265-017.
Jacobs, P.P., Geysens, S., Vervecken, W., Contreras, R., and Callewaert, N. 2008. Engineering complex-type N-glycosylation in Pichia pastoris using GlycoSwitch technology. Nat. Protocols 4, 58–70.
Kakkis, E.D., Muenzer, J., Tiller, G.E., Waber, L., Belmont, J., Passage, M., Izykowki, B., Philips, J., Doroshow, R., Walot, I., and et al. 2001. Enzyme-replacement therapy in mucopolysaccharidosis I. New Eng. J. Med. 344, 182–188.
Kaplan, W., Husler, P., Klump, H., Erhardt, J., Sluis-Cremer, N., and Dirr, H. 1997. Conformational stability of pGEX-expressed Schistosoma japonicum glutathione S-transferase: A detoxification enzyme and fusion-protein affinity tag. Prot. Sci. 6, 399–406.
La Vallie, E.R. and McCoy, J.M. 1994. Enzymatic cleavage of fusion proteins with factor Xa. John Wiley & Sons, pp. 16.14.16–16.14.17. New York, N.Y., USA.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Landázuri, P. 2002. Clonación Del cDNA de la Iduronato 2-Sulfato Sulfatasa Humana. Expresión de la Enzima en Escherichia coli y Pichia pastoris, p. 134. Tesis Doctoral Thesis, Pontificia Universidad Javeriana, Bogotá, Columbia.
Landázuri, P., Poutou-Piñales, R.A., Acero-Godoy, J., Córdoba-Ruiz, H.A., Echeverri-Peña, O.Y., Sáenz, H., Delgado-Boada, J.M., and Barrera-Avellaneda, L.A. 2009. Cloning and shake flask expression of hrIDS-Like in Pichia pastoris. Afr. J. Biotechnol. 8, 2871–2877.
Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., López, R., Thompson, J.D., Gibson, T.J., and Higgins, D.G. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948.
Lili, W., Chaozhan, W., and Xindu, G. 2006. Expression, renaturation and simultaneous purification of recombinant human stem cell factor in Escherichia coli. Biotechnol. Lett. 28, 993–997.
Lissens, W., Zenati, A., and Liebaers, I. 1984. Polyclonal antibodies against iduronate 2-sulfatase from human urine. Biochem. Biophys. Acta. 801, 365–371.
Majidzadeh-A, K., Mahboudi, F., Hemayatkar, M., Davami, F., Barkhordary, F., Adeli, A., Soleimani, M., Davoudi, N., and Khalaj, V. 2010. Human tissue plasminogen activator expression in Escherichia coli using cytoplasmic and periplasmic cumulative power. Avicenna J. Med. Biotechnol. 2, 131–136.
Maleki, A., Roohvand, F., Tajerzadeh, H., Khanahmad, H., Nobari, M.B., Beiruti, A., and Najafabadi, A.R. 2010. High expression of methylotrophic yeast-derived recombinant human erythropoietin in a pH-controlled batch system. Avicenna J. Med. Biotechnol. 2, 197–206.
Meza, R.A., Monroy, A.F., Mercado, M., Poutou, R.A., Rodríguez, P., and Pedroza, A.M. 2004. Study of the stability in real time of cryopreserved strain banks. Univ. Sci. 9, 35–42.
Miech, C., Dierks, T., Selmer, T., von Figura, K., and Schmidt, B. 1998. Arysulfatase from Klebsiella pneumoniae carries a formylglycine generated from serine. J. Biol. Chem. 273, 4835–4837.
Millat, G., Froissart, R., Maire, I. and Bozon, D. 1997. Characterization of iduronate sulfate sulfatase mutants affecting N-glycosilations sites and cysteine-84 residue. Biochem. J. 326, 243–247.
Muenzer, J., Gucsavas-Calikoglu, M., McCandless, S.E., Schuetz, T.J., and Kimura, A. 2007. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome). Mol. Genet. Metab. 90, 329–337.
Muenzer, J., Wraith, J.E., Beck, M., Giugliani, R., Harmatz, P., Eng, C.M., Vellodi, A., Martin, R., Ramaswami, U., Gucsavas-Calikoglu, M., and et al. 2006. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Gen. Med. 8, 599.
Nabavinia, M.S., Nasab, M.N., Meshkat, Z., Derakhshan, M., and Khaje-Karamadini, M. 2011. Construction and evaluation of an expression vector containing Mtb32C (Rv0125) of Mycobacterium tuberculosis. Avicenna J. Med. Biotechnol. 3, 207–210.
Nagai, K. and Thøgersen, H.C. 1984. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. Nature 309, 810–812.
Nagai, K. and Thøgersen, H.C. 1987. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Meth. Enzymol. 153, 461–481.
Neufeld, E.F. and Muenzer, J. 2001. The mucopolysaccharidoses. In Scriver, C.R., Beaudet, A.L., Sly, W., and Valle, D. (eds.), The metabolic and molecular bases of inherited disease, pp. 3421–3452. McGraw-Hill, New York, N.Y., USA.
Niederau, C., Vom Dahl, S., and Haussinger, D. 1998. First long-term results of imiglucerase therapy of type 1 Gaucher disease. Eur. J. Med. Res. 3, 25–30.
Nyhan, W.L. and Ozand, P.T. 1998. Hunter disease/mucopolysaccharidoses type II (MPSII) iduronate sulfatase deficiency, Atlas of Metabolic Diseases, pp. 455–461. Chapman and Hall Medical, New York, USA.
Parenti, G., Meroni, G. and Ballabio, A. 1997. The sulfatase gene family. Curr. Opin. Gen. Devel. 7, 386–391.
Peña, O., Sosa, A., Echeverri, O., Sáenz, H., and Barrera, L.A. 2005. Producción de anticuerpos policlonales IgG contra la proteína Iduronato-2-sulfato sulfatasa y desarrollo de un sistema de detección para IDS humana recombinante. Biomédica. 25, 181–188.
Poutou, R.A. 2006. Expresión de sulfatasas humanas en Escherichia coli y Pichia pastoris, p. 162. Tesis Doctoral Thesis, Pontificia Universidad Javeriana, Bogotá, D.C., Columbia.
Poutou, R.A., Córdoba, H., Quevedo, B.E., Landázuri, P., Echeverri, O.Y., Sáenz, H., Vanegas, A., Acero, J., Gónzalez, A., Herrera, J., and et al. 2005. Expresión de iduronato 2-sulfato sulfatasa humana recombinante (IDShr) en Pichia pastoris. Univ. Sci. 10, 75–96.
Poutou-Piñales, R.A., Vanegas Ni~no, A., Landázuri, P., Sáenz, H., Lareo, L., Echeverri, O.Y., and Barrera Avellaneda, L.A. 2010. Human sulfatase transiently and functionally active expressed in E. coli K12. Elect. J. Biotechnol. 13, article 8.
Rodríguez, A., Espejo, Á.J., Hernández, A., Velásquez, O.L., Lizaraso, L.M., Cordoba, H.A., Alméciga-Díaz, C., Barrera, L.A., and Sanchez, O. 2010. Enzyme replacement therapy for Morquio A: an active recombinant N-acetylgalactosamine-6-sulfate sulfatase produced in Escherichia coli BL21. J. Ind. Microbiol. Biotechnol. 37, 1193–1201.
Sáenz, H. 2005. Expresión, purificación parcial y estudios computacionales de la IDShr producida en Pichia pastoris, p. 180. Doctoral Thesis, Pontificia Universidad Javeriana, Bogotá, D.C., Colombia.
Sáenz, H., Lareo, L., Poutou, R.A., Sosa, C., and Barrera, L.A. 2007. Predicción computacional de la estructura terciaria de la iduronato 2-sulfato sulfatasa humana. Biomédica. 27, 7–20.
Saluta, M. and Bell, P. 2005. Troubleshooting GST fusion protein expression in E. coli. Amersham Biosciences, Piscataway, NJ, USA.
Sambrook, J. and Russell, D.W. 2001. Molecular cloning: A laboratory manual, p. 2100. Cold Spring Harbor Laboratory Press, New York, N.Y., USA.
Scott, H.S., Blanch, L., Guo, X.-H., Freeman, C., Orsborn, A., Baker, C., Sutherland, G.R., Morris, C.P. and Hopwood, J.J. 1995. Cloning of the sulphamidase gene and identification of mutations in Sanfilippo A syndrome. Nat. Genet. 11, 465–467.
Söding, J. 2005. Protein homology detection by HMM-HMM comparison. Bioinformatics 21, 951–960.
Sosa, A.C., Espejo, A.J., Rodriguez, E.A., Lizaraso, L.M., Rojas, A., Guevara, J., Echeverri, O.Y., and Barrera, L.A. 2011. Development of a sandwich enzyme linked immunosorbent assay (ELISA) for the quantification of iduronate-2-sulfate sulfatase. J. Immunol. Methods 368, 64–70.
Szameit, C., Miech, C., Balleinninger, M., Bernhard., S., von Figura, K., and Dierks, T. 1999. The iron sulfur protein AtsB is required for posttranslational formation of formylglycine in the Klebsiella sulfatase. J. Biol. Chem. 274, 15375–15381.
Vervecken, W., Kaigorodov, V., Callewaert, N., Geysens, S., De Vusser, K., and Contreras, R. 2004. In vivo synthesis of mammalian-like, hybrid-type N-glycans in Pichia pastoris. Appl. Environ. Microbiol. 70, 2639–2646.
Vincze, T., Posfai, J., and Roberts, R.J. 2003. NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acid Res. 30, 3688–3691.
von Figura, K., Bemhard, S., Thorsten, S., and Dierks, T. 1998. A novel protein modification generating an aldehyde group in sulfatases: Its role in catalysis and disease. BioEssay 20, 505–510.
von Figura, K., Gieselman, V., and Jaeken, J. 2001. Metachromatic leukodystrophy. In Scriver, C.R., Beaudet, A., Sly, W., and Valle, D. (eds.), The metabolic and molecular bases of inherited disease, pp. 3695–3724. Mc Graw-Hill, New York, N.Y., USA.
Voznyi, Y.V., Keulemans, L.M., and van Diggelen, O.P. 2001. A fluorogenic enzyme assay for the diagnosis of MPS II (Hunter disease). J. Inherit. Metabol. Dis. 24, 675–680.
Waldow, A., Schmidt, B., Dierks, T., von Bülow, R., and von Figura, K. 1999. Amino acids residues forming the active site of arylsulfatase A. J. Biol. Chem. 274, 12284–12288.
Weaston, A. and Neufeld, E.F. 1982. Iduronate of human plasma. Meth. Enzymol. 83, 573–578.
Weinreb, N., Charrow, J., Anderson, H.C., Kaplan, P., Kolodny, E.H., Mistry, P., Pastores, G., Rosenbloom, B.E., Ronald, C., Wappner, R., and et al. 2002. Eficacia de la terapia de reemplazo enzimático en 1.028 pacientes con enfermedad de Gaucher tipo 1 después de 2 a 5 a.os de tratamiento: Comunicación del Gaucher registry. Amer. J. Med. 113, 1–8.
Zhao, Y., Liu, Z., Yu, S., Wen, S., Hammarstrom, L., and Rabbani, H. 2009. Construction of a high efficiency PCR products cloning T vector using pGEM-5zf (+). Avicenna J. Med. Biotechnol. 1, 37–39.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Morales-Álvarez, E.D., Rivera-Hoyos, C.M., Baena-Moncada, A.M. et al. Low-Scale expression and purification of an active putative iduronate 2-sulfate sulfatase-Like enzyme from Escherichia coli K12. J Microbiol. 51, 213–221 (2013). https://doi.org/10.1007/s12275-013-2416-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-013-2416-2