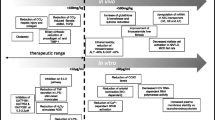
Abstract
Silymarin, an extract from milk thistle seeds, has been used for centuries to treat hepatic conditions. Preclinical data indicate that silymarin can reduce oxidative stress and consequent cytotoxicity, thereby protecting intact liver cells or cells not yet irreversibly damaged. Eurosil 85® is a proprietary formulation developed to maximize the oral bioavailability of silymarin. Most of the clinical research on silymarin has used this formulation. Silymarin acts as a free radical scavenger and modulates enzymes associated with the development of cellular damage, fibrosis and cirrhosis. These hepatoprotective effects were observed in clinical studies in patients with alcoholic or non-alcoholic fatty liver disease, including patients with cirrhosis. In a pooled analysis of trials in patients with cirrhosis, silymarin treatment was associated with a significant reduction in liver-related deaths. Moreover, in patients with diabetes and alcoholic cirrhosis, silymarin was also able to improve glycemic parameters. Patients with drug-induced liver injuries were also successfully treated with silymarin. Silymarin is generally very well tolerated, with a low incidence of adverse events and no treatment-related serious adverse events or deaths reported in clinical trials. For maximum benefit, treatment with silymarin should be initiated as early as possible in patients with fatty liver disease and other distinct liver disease manifestations such as acute liver failure, when the regenerative potential of the liver is still high and when removal of oxidative stress, the cause of cytotoxicity, can achieve the best results.
Similar content being viewed by others
Silymarin-Eurosil 85 is a formulation of silymarin with high oral bioavailability and potent antioxidant effects in preclinical models of liver disease. |
Silymarin acts as a free radical scavenger, along with modulating the enzymes responsible for the development of cellular damage, fibrosis and cirrhosis. |
Clinically, silymarin reduces liver dysfunction, may reduce liver-related mortality in patients with cirrhosis and improves glycemic control in patients with concomitant diabetes, with few if any adverse events. |
By reducing oxidative stress and consequent cytotoxicity, silymarin protects intact liver cells or cells not yet irreversibly damaged and thus may be considered to be hepatoprotective. |
For maximum benefit, silymarin should be initiated as early as possible in patients with fatty liver disease when the regenerative potential of the liver is still high. |
Introduction
Worldwide, approximately 2 million people a year die as a result of liver diseases, with cirrhosis (the 11th most common cause of mortality) causing approximately 1.16 million of these deaths [1]. In Western industrialized countries, the leading causes of cirrhosis are now alcohol and non-alcoholic fatty liver disease (NAFLD), while hepatitis B is still one of the main causes in many Asian counties [1].
The therapeutic use of natural components has received considerable attention in the last 2 decades. Silybum marianum (milk thistle) has been safely used for centuries as a natural herbal medicine for the treatment of liver disorders. The bioactive extract of milk thistle, silymarin, has well-documented antioxidant and hepatoprotective properties in preclinical studies [2,3,4,5,6,7]. A number of silymarin formulations are available, including Legalon®, which contains the Eurosil 85® formulation that has high oral bioavailability and well-characterized pharmacokinetic and pharmacodynamic properties. Most clinical data discussed in this review have been obtained using the Legalon® formulation. The aim of the current narrative review is to describe the pharmacologic features of silymarin extract and to review the data surrounding its use as supportive treatment in patients with liver diseases.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Silymarin Pharmacology
Chemistry
Silymarin is an extract from the dried seeds and fruits of the milk thistle plant (S. marianum). Milk thistle has been used medicinally in Europe since the first century AD. Its medicinal properties were mentioned in the writings of the Greek physician and botanist Dioscorides (40–90 AD), who recommended it as a treatment for snakebite [8, 9]. The sixteenth century English herbalist Nicholas Culpeper recommended milk thistle for jaundice and for expelling stones [9]. By the nineteenth century, a German scientist, Johannes Gottfried Rademacher, had shown that extracts or ‘tinctures’ from milk thistle seeds were beneficial for treating patients with liver disorders [9, 10].
The milk thistle extract silymarin is a complex mixture of plant-derived compounds identified as mostly flavonolignans, flavonoids (taxifolin, quercetin) and polyphenolic molecules [11]. These compounds are known to be antioxidants in addition to having several other biologic properties [12]. The four main flavonolignan isomers in silymarin are silibinin, isosilibinin, silichristin and silidianin, but the most prevalent and biologically active of these is silibinin (also called silybin). Approximately 50–60% of the silymarin complex is silibinin, with the other flavonolignan isomers comprising about 35%: silichristin (~ 20%), silidianin (~ 10%) and isosilibinin (~ 5%) [13, 14].
Silibinin is a polyphenolic flavonoid antioxidant with the molecular formula of C25H22O10 and with a molecular weight of 482.44 g/mol [15]. Silibinin itself is mixture of two diastereomers, silibinin A and silibinin B, in an approximately equimolar ratio (Fig. 1) [16]. It undergoes phase I and phase II biotransformation in the liver. During phase II, multiple conjugation reactions have been observed that include the formation of glucuronide and glucuronide sulfate derivatives [11, 17].
Silymarin was first isolated in 1968 by German scientists at the University of Munich and then described and patented by the German herbal medicine manufacturer Madaus as a specific treatment “against liver diseases” [10]. The first commercial preparation of silymarin was developed by Rottapharm/Madaus (Cologne, Germany) and complies with the analytical specifications reported in the European Pharmacopoeia 01/2005 under “Milk Thistle fruit.” It is registered as a drug for liver diseases in many countries in Europe, Asia, America, Africa and Australia. Different forms, including capsules and tablets, are available with different dosages; the recommended daily dosage (depending on the commercial formulation used) is between 420 and 600 mg, and the majority of clinical trials have been conducted with a dosage of 140 mg three times a day.
Pharmacokinetics
Crude silymarin extract is lipophilic and poorly soluble in water, so only about 20–50% is absorbed from the gastrointestinal tract after ingestion [18, 19]. For this reason, formulation scientists have endeavored to improve the oral bioavailability and solubility of silymarin preparations, but the commercially available silymarin-containing products differ significantly in their content, dissolution and oral bioavailability of the active ingredient silibinin [20]. In 1995, Rottapharm/Madaus invented a co-precipitation processing method that produced a high-quality silymarin (90–96% purity; approximately 60% of the content being silibinin) with an enhanced dissolution profile (> 90% of silibinin liberated by the co-precipitate); this advanced processing method was subsequently patented in 2014 under the trade name Eurosil 85® [20,21,22]. Most of the published clinical research on silymarin has used this standardized pharmaceutical preparation.
The silymarin formulation derived using the Eurosil 85® extraction method contains 60% silibinin and has a bio-dissolution of up to 85%. Therefore, the commercially available silymarin capsule, at a daily dosage of 3 capsules, provides 420 mg of silymarin, corresponding to 250 mg of silibinin [23].
Silymarin from this specific orally administered formulation is rapidly absorbed; the peak plasma concentration of silibinin is reached about 2–4 h after oral administration, and its plasma half-life is approximately 6 h [23]. It has been established that 3–7% of orally administrated silibinin is excreted in an unchanged form in the urine [24]. After gastrointestinal absorption silibinin and the other components of silymarin are rapidly metabolized by phase I and phase II biotransformation reactions in liver cells [11] and undergo extensive enterohepatic circulation [23]: about 80% of silibinin is excreted as glucuronide and sulfate conjugates with bile [25, 26]. It is assumed that 20–40% of bile silibinin is recovered, whereas the remaining part is excreted via feces [27, 28].
Silymarin was assessed for drug–drug interaction and for cytochrome P450 (CYP450) induction or inhibition by permeability studies with Caco-2 cells and by studies with human primary hepatocytes and with human liver microsomes, respectively [29]. At a supratherapeutic concentration (1 µmol/l), there was negligible inhibition of the CYP450 enzymes 1A2, 2A6, 2B6, 2C8, 2C9 and 2E1, minor (< 20%) inhibition of CYP 3A4 and moderate (< 40%) inhibition of CYP 2C19 and 2D6. The authors concluded that, since the therapeutic concentration of silibinin is ~ 0.2 µmol/l, silymarin is unlikely to cause hepatic drug–drug interactions at the standard dose [29]. Results of trials in healthy volunteers and/or clinical trials suggest that milk thistle does not affect CYP 1A2, 2C9, 2D6, 2E1, 3A4 or 3A5 [30]. In two multiple-dose pharmacokinetic studies, silymarin (160–450 mg every 8 h) did not reduce levels of the CYP 3A4 substrate indinavir [30]. However, as our knowledge in this area is incomplete, patients taking silymarin along with CYP450 enzyme substrates should be advised to watch for signs of drug–drug interactions [30].
Because silymarin has been shown to lower elevated blood glucose and hemoglobin A1c levels in patients with diabetes, there is theoretical potential for an additive risk of hypoglycemia in patients taking antidiabetic drugs [30]. However, there is no documented hypoglycemia and no clinical evidence of this additive effect. Other theoretical drug interactions with silymarin, based on laboratory/animal studies, include inference with estrogen therapy (in animal studies, silymarin binds to estrogen receptor beta), reduced clearance of glucuronidated drugs (in laboratory studies, milk thistle inhibited uridine diphosphoglucuronosyl transferase) and increased absorption of P-glycoprotein substrates (in vitro, milk thistle can inhibit P-glycoprotein activity) [30]. Silymarin and silibinin have the potential to interact with statins; in vitro they inhibit both organic anion transporting polypeptide 1B1 (transports statins into the liver) and breast cancer resistance protein (transports statins from the liver to the bile) [30]. However, silymarin (140 mg, 3 times a day) did not alter the pharmacokinetics of a single 10-mg dose of rosuvastatin in a study in healthy males [30]. In a trial in hepatically impaired renal transplant patients, silymarin reduced the apparent clearance of the immunosuppressant sirolimus [30].
Pharmacodynamics
Several pharmacologic actions of silibinin have been identified including antioxidant properties, anti-inflammatory properties, antifibrotic effects and insulin resistance modulation.
Antioxidant Properties
The production of reactive oxygen species (ROS) is a natural consequence of a variety of essential biochemical reactions in the liver, mostly related to the processes involved in detoxification. Exposure to high levels of toxins (e.g., alcohol, hepatotoxic drugs) or intensive oxidation of free fatty acids (i.e., insulin resistance) leads to abnormal production of ROS; the endogenous antioxidants may also become depleted. For example, it is widely acknowledged that ethanol promotes the formation of various free radicals in several cell types, including hepatocytes, Kupffer cells, endothelial cells and infiltrating inflammatory leukocytes [31]. The consequent imbalance, with persistent presence of ROS that are not neutralized by endogenous antioxidants, creates a condition called “oxidative stress”, which is implicated in the pathogenesis of a variety of liver disorders including liver fibrosis [32].
In vitro, silibinin is found to be a potent scavenger of ROS, such as hydroxyl and peroxyl anions and hypochlorous acid, in various model systems, such as rat liver microsomes [6], as well as human platelets, leukocytes, endothelial cells [33], erythrocytes [34] and fibroblasts [35]. In addition, superoxide anion radicals and nitric oxide were inhibited in isolated Kupffer cells after treatment with silibinin (concentration at which 50% inhibition occurs of 80 μmol/l) [2].
Silymarin may augment the generation of glutathione in the liver via an increase in substrate availability (i.e. cysteine) for its biosynthesis, which subsequently contributes to the enhancement of its antioxidant capacity in liver tissues [3].
Silymarin protects liver cells by a number of mechanisms. First, it stabilizes membrane permeability through inhibition of lipid peroxidation, thereby helping the liver to maintain levels of its own protective antioxidant, glutathione [3]. Silymarin also protects against injury from various toxic chemicals such as carbon tetrachloride [36], for example, by inhibiting the production of tumor necrosis factor-alpha (TNF-α), interferon-gamma, interleukin (IL)-2 and IL-4 [36, 37] as a consequence of blocking hepatic nuclear factor kappa B (NFκB) activation [36, 38]. Silymarin is able to reduce the cellular uptake of xenobiotics, including mushroom poisons, by blocking organic ion uptake transporters on the surface of hepatocytes [39]. It also inhibits TNF-α expression, for example, when induced by α-amanitin toxin from poisonous mushrooms [40]. The hepatoprotective properties of silibinin are widely attributed to these antioxidant activities [41].
Anti-Inflammatory Properties
Chronic inflammation has been associated with progressive hepatic fibrosis and the development of cirrhosis [42], and oxidative stress may be the common underlying mechanism in the initiation and progression of hepatic inflammation in various liver disorders [10]. NF-κB is an important transcriptional regulator of the inflammatory response and plays an essential role in regulating inflammatory signaling pathways in the liver [43]. Moreover, NF-κB is activated in virtually every chronic liver disease, including AFLD [44], NAFLD [45], viral hepatitis [46] and biliary liver disease [47, 48]. There is increasing evidence that demonstrates the overall inhibition by silymarin of inflammatory mediators such as NF-κB and inflammatory metabolites (e.g., prostaglandin E2 [PGE2] and leukotriene B4 [LTB4]) [49].
Kupffer cells are resident liver macrophages that appear to be involved in innate immune responses and host defense through the expression and secretion of inflammatory mediators [50]. In isolated rat Kupffer cells, silymarin weakly inhibited PGE2 formation but strongly inhibited LTB4 formation, even at low concentrations (15 μmol/l) [2]. This selective inhibition of LTB4 formation by Kupffer cells and possibly other cell types may account for the anti-inflammatory potential of silymarin.
Antifibrotic Effects
Silibinin has demonstrated antifibrogenic effects in animal and in vitro models [38, 49, 51]. Hepatic fibrogenesis, which results from chronic liver tissue damage, is characterized by activation of hepatic stellate cells (HSCs), a liver-specific type of pericyte. Activated HSCs develop into myofibroblasts, which are responsible for the deposition of collagen fibers leading to liver cirrhosis. In an in vitro model of human hepatic fibrogenesis, silibinin demonstrated antifibrogenic properties by dose-dependently inhibiting the growth factor-induced production of pro-collagen in activated human HSC [38].
The antifibrogenic effect of silymarin has also been confirmed in an animal model of alcohol-induced hepatic fibrosis in non-human primates receiving chronic treatment with alcohol [49]. In this study, baboons were fed alcohol (50% of daily calories) for 3 years with a nutritionally adequate diet, which resulted in an increase of collagen type I in hepatic biopsy samples. Results showed that concomitant administration of silymarin significantly reduced the alcohol-induced increase in hepatic collagen type I (Fig. 2) [49].
Modulation of Insulin Resistance
Insulin resistance is widely recognized as the key mechanism in the pathogenesis of NAFLD. In a rat model of NAFLD, silibinin ameliorated insulin resistance by reducing visceral obesity, enhancing lipolysis and inhibiting gluconeogenesis [52].
Clinical Effects of Silymarin
Liver Cirrhosis/Alcohol-Related Liver Disease
Fatty liver disease (FLD) is caused by the accumulation of excess fat in the liver, which can lead to serious liver disease for many people. In individuals who consume too much alcohol, alcoholic fatty liver disease (AFLD) is the earliest stage of alcoholic-related liver disease [53, 54]. Silymarin has been investigated in a number of clinical studies in patients with liver cirrhosis and/or alcohol-related liver disease (Table 1) [55,56,57,58,59,60,61,62,63]. Six of these clinical trials were conducted in patients affected by liver cirrhosis (mainly alcohol-related) [55,56,57,58,59,60]. Four studies examined the impact of silymarin on clinical outcomes such as mortality [55, 57, 58, 60], and two of these trials had survival as the primary clinical endpoint [55, 57]. The impact of silymarin in these studies is shown in Table 2, with the study by Ferenci et al. showing a significant impact on mortality [55]. This was a double-blind, prospective, randomized study that was performed to determine the effect of silymarin (Eurosil 85®-derived formulation) on the outcome of patients with cirrhosis [55]. Of the 170 patients with cirrhosis, 87 were treated with silymarin 420 mg/day (alcoholic: 47, non-alcoholic: 40), and 83 received placebo (alcoholic: 45, non-alcoholic: 38) for at least 24 months, with a median observation period of 41 months. In the placebo group, there were 32/39 liver-related deaths, whereas in the silymarin group 16/28 patient deaths were related to liver disease. In this study, the 4-year survival rate was significantly higher (58% vs. 39%) in silymarin recipients than placebo recipients (P = 0.036) [55]. Subgroup analyses found that treatment reduced mortality in patients with alcoholic cirrhosis (P = 0.01) and in patients with less severe cirrhosis (class A disease according to the Child-Turcotte criteria [64]) (P = 0.03).
In another similar randomized controlled trial by Pares et al. [57], survival was investigated in patients receiving the specific Eurosil 85®-derived oral formulation of silymarin or placebo over 2 years. The mortality rate was 14.6% in the silymarin group and 14.4% in the placebo group (not statistically significant over this shorter duration of treatment). However, in a subgroup analysis of patients with a diagnosis of hepatitis C (29/75 patients), no deaths occurred in the silymarin group (0/13) while 4/16 patients in the placebo group died (P = 0.06) [57].
A review of clinical data with silymarin calculated the overall odds ratio for liver-related mortality in the silymarin versus placebo groups across the five studies as 0.53 (i.e., 47% risk reduction; 95% confidence intervals 0.33–0.86) [13]. In this analysis, the pooled liver-related mortality rate was 4.9% per year in patients receiving silymarin compared with 9.3% per year in patients receiving placebo [13]. This review also noted that in one of the studies, the proportion of patients requiring hospital admission because of liver-related complications was lower in those receiving silymarin than in those receiving placebo (10.0% vs. 16.3%; P < 0.01) [60]. Therefore, the lack of an effect of silymarin on survival in three of the four studies with death as an outcome [57, 58, 60] may have been because the trials were underpowered, not long enough or included too many patients with severe/advanced disease to be able to demonstrate an impact on mortality. A Cochrane review of trials in patients with alcoholic and/or viral liver disease found that, compared with placebo or no intervention, milk thistle significantly reduced liver-related mortality in all reviewed trials, but not when the analysis was limited to high-quality trials [65]. Further large-scale studies are warranted to clarify the effect of silymarin on liver-related mortality.
Where liver function was examined, silymarin consistently demonstrated a reduction in liver enzyme levels [alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels] compared with placebo [60,61,62]. For example, in a randomized study in 97 patients with histologically diagnosed mild, acute and subacute liver disease induced by alcohol abuse, silymarin treatment for 4 weeks resulted in a significantly greater improvement in liver function, as evidenced by a decrease in ALT and AST levels, compared with placebo [61].
Several studies also demonstrated an improvement in oxidative stress parameters [56, 59, 63]. For example, a double-blind, placebo-controlled study in patients with chronic alcoholic liver disease showed that 6 months of treatment with silymarin (Eurosil 85®-derived formulation) significantly restored the antioxidant defense system, as indicated by the following: increased superoxide dismutase activity of erythrocytes and lymphocytes, increased free-SH group serum levels and increased glutathione peroxidase activity (Fig. 3) [63]. In a 12-month randomized controlled study, 60 insulin-treated diabetic patients with alcoholic cirrhosis were treated with either silymarin 600 mg/day plus standard therapy or standard therapy (control group) [59]. The aim of this study by Velussi et al. [59] was to ascertain whether long-term treatment with silymarin was effective in reducing lipoperoxidation and insulin resistance in diabetic patients with liver cirrhosis. Results showed that silymarin effectively neutralized excess superoxides and reduced systemic signs of inflammation (C-peptide levels) [59]. In addition to reducing membrane peroxidation, silymarin also significantly reduced levels of glycosylated hemoglobin and insulin requirements (Fig. 4) [59].
Effect of silymarin on antioxidant capacity [63]. This was measured by serum levels of free sulfhydryl groups and glutathione peroxidase activity in erythrocytes and lymphocytes in patients with alcohol-induced liver disease receiving silymarin or placebo. SH: sulfhydryl; GSH-Px: glutathione peroxidase. aP < 0.05 vs. month 0; bP < 0.05 vs. placebo [63]

Reproduced with permission from Velussi et al. [59]
Changes in alcoholic liver disease and diabetes parameters in patients with diabetes and alcoholic liver disease receiving standard treatment alone (untreated) or with concomitant silymarin (treated) [59]. a Plasma malondialdehyde levels (a marker of membrane peroxidation); b glycosylated hemoglobin; c average daily insulin dose. aP < 0.05 vs. untreated control group; bP < 0.01 vs. untreated control group.
NAFLD and Non-Alcoholic Steatohepatitis
NAFLD is another major cause of chronic liver disease in the absence of significant alcohol consumption and is frequently associated with insulin resistance, central obesity, type-2 diabetes and dyslipidemia [42, 66]. Due to the exploding prevalence of these comorbidities, NAFLD is recognized as a major worldwide health problem and the leading cause of liver disease in Western countries with a prevalence up to 33% [67]. There is also an increasing prevalence of NAFLD in Eastern countries, reflecting the increasing incidence of obesity and obesity-related diseases in these regions [68].
NAFLD encompasses a wide spectrum of disorders, ranging from benign fat accumulation (simple steatosis), with or without varying degrees of hepatic inflammation (steatohepatitis), to progressive fibrosis and ultimately to cirrhosis and end-stage liver disease [66, 69]. Approximately 20% of patients with NAFLD (simple steatosis) will go on to develop the more severe form known as non-alcoholic steatohepatitis (NASH) [70].
To date, the combination of dietary modifications and increased physical activity remains the mainstay of NAFLD management [71]. Unfortunately, however, many patients find instituting lifestyle changes difficult over the long term [72].
Oxidative stress is regarded as the key pathogenic component involved in the progression of simple steatosis to NASH [73]. Endogenous antioxidants function as direct scavengers of ROS and are thus able to either delay or prevent oxidative stress as well as other parameters of hepatocyte damage [74,75,76]. Glutathione is the most abundant cellular antioxidant that protects hepatocytes against the toxic effects of ROS [77, 78]. Recent literature strongly suggests that treatment with antioxidant agents and other putative free radical scavengers is beneficial in improving biochemical and histologic parameters in NASH [67, 79].
Use of vitamin E as an antioxidant has led to controversial results; it has been investigated as a treatment for NASH or NAFLD in two large randomized clinical trials [80, 81]. Vitamin E therapy, as compared with placebo, was associated with a significantly higher rate of improvement in adult patients with NASH, but only at a very high dosage (533.6 mg/day for 96 weeks) [81]. Use at such high dosage over a prolonged period has raised concerns about the long-term safety of vitamin E, particularly in patients with NAFLD who have not yet progressed to NASH [71]. The widespread use of vitamin E in all NAFLD patients is not currently recommended and should be limited to non-diabetic patients with biopsy-proven NASH [71].
Silymarin (Eurosil 85®-derived formulation) has been studied as a treatment option for NAFLD and NASH (Table 3) [79, 82, 83]. The pilot study by Butorova et al. was conducted in patients with either NAFLD or NASH, who were treated for 2 months with diet only or with silymarin [82]. These results indicated that silymarin was able to reduce or normalize liver function parameters (transaminase levels) and improve ultrasound parameters of liver anatomy [82].
Another study involved patients with NAFLD treated for 3 months with either diet or diet plus a novel formulation of silymarin plus vitamin E in a dietary supplement [83] (same daily posology of silymarin as Silymarin-Eurosil 85®). Validated indices of liver steatosis (e.g., lipid accumulation product, hepatic steatosis index) were used as outcome parameters. Results showed significant improvements of both indices together with improvements in biometric parameters (e.g., abdominal circumference, body mass index) in the group receiving silymarin/vitamin E compared with the placebo group. These findings suggest that, in patients with uncomplicated NAFLD, for whom the standard treatment would be limited to diet and exercise only, the use of silymarin/vitamin E as a dietary adjunct is potentially more effective than diet alone and may possibly improve patient motivation to sustain lifestyle changes over time [83].
Since pilot studies showed only a trend towards improvement when patients with NASH were treated with the commercial (Eurosil 85®) formulation of silymarin at the currently approved dosage of 420 mg/day, another clinical study was conducted with a higher dosage of silymarin. Previous phase I studies had shown that silymarin was safe at doses of up to 2100 mg/day, so this dosage was chosen to treat patients with biopsy-confirmed NASH for 48 weeks in a randomized, double-blind, placebo-controlled trial [79]. Although no statistically significant difference was reached for the primary endpoint [≥ 30% improvement from baseline in the NASH and NAFLD Activity Score (NAS) on liver biopsy], significantly more patients in the silymarin than the placebo group had a measurable improvement in fibrosis (Fig. 5a). In addition, there were more patients with fibrosis improvement or resolution of fibrosis in the silymarin group, and the change in liver stiffness favored silymarin (change in liver stiffness − 0.7 vs. 6.0 kPa), although between-group differences were not significant [79]. Improvements in noninvasive markers of fibrosis (AST to platelet ratio index, fibrosis-4 score and NAFLD fibrosis score) were observed in the group receiving silymarin but not in the group receiving placebo (Fig. 5b) [79].

Part B of figure reproduced with permission from Wah Kheong et al. [79]
Effect of 48 weeks’ treatment with silymarin 2100 mg/day in 99 patients with histologically proven non-alcoholic steatohepatitis [79]. A proportion of patients with: a NAS score improvement (defined as ≥ 30% improvement in NAS), fibrosis improvement (defined as a ≥ 1 point improvement in the histologic component of the NAS score), resolution of fibrosis (defined as absence of fibrosis at EOT) or development of cirrhosis [79]. aP < 0.05 vs. placebo; bline charts illustrating the changes in the (i) APRI, (ii) FIB-4 score and (iii) NAFLD fibrosis scores in the silymarin and placebo groups [79]. APRI aspartate aminotransferase to platelet ratio index, EOT end of treatment, FIB-4 fibrosis-4, NAFLD nonalcoholic fatty liver disease, NAS NASH and NAFLD activity score, NASH non-alcoholic steatohepatitis.
Amatoxin-Induced Liver Failure
The ingestion of amatoxin-containing mushrooms may result in hyperacute liver failure, depending on the ingested dose [84, 85]. Amatoxin is known to inhibit RNA polymerase II [85], which is essential for hepatocyte function. Therefore, amatoxin is used experimentally as a toxic model for liver failure. Although no prospective studies on the use of silymarin for amatoxin-induced liver failure in mushroom poisoning can be designed, abundant clinical evidence shows that parenteral use of a silibinin-based formulation may be considered as the treatment of choice in this setting [84, 86]. Early diagnosis and prompt initiation of intravenous therapy are crucial.
Drug-Induced Liver Injury
It is well known that many drugs undergo hepatic metabolism and can induce, directly or through their active metabolites, hepatotoxicity. As has been observed with anti-tuberculosis drugs (ATDs), this may result in increased morbidity or mortality [87]. Hepatotoxicity may necessitate treatment discontinuation, drug interruption and substitution or dose adjustment [87]. Drug-induced liver injury (DILI) is still the most common cause of acute liver failure in Western societies [88].
Previous studies have reported that certain herbal drugs, phytochemicals and food supplements can prevent and reduce the hepatotoxicity of different drugs [89].
Several trials have investigated the effectiveness of silymarin in preventing DILI from ATDs. In a prospective, multicenter trial, patients (N = 565) were randomized to receive ATDs and silibinin capsules (70 mg, 3 times a day) or ATDs only [90]. After 8 weeks of therapy, there were no significant differences between patients receiving silibinin and controls in the number of patients with liver injury (2.2% vs. 2.4%), diagnosed with DILI (7.2% vs. 9.3%) or who had ATD treatment that was suspended because of liver injury and symptoms (3.25% vs. 6.19%). However, fewer patients in the silibinin group experienced the liver injury symptoms anorexia and nausea (P < 0.05) [90]. In contrast, in another smaller trial (N = 55), after 4 weeks of treatment the incidence of ATD DILI was 3.7% with silymarin compared with 32.1% with placebo [91]. The decline in levels of the antioxidant enzyme superoxide dismutase was also significantly lower with silymarin than placebo, and the authors attributed the lower risk of liver injury to superoxide dismutase restoration [91]. A recent meta-analysis, which included a total of 1198 patients from five randomized controlled trials [n = 585 (silymarin); n = 613 (placebo)] concluded that prophylactic therapy with silymarin contributed to a noticeably reduced risk of development of ATD DILI 4 weeks after the initiation. In addition, silymarin significantly improved liver function, measured by a reduction in ALT, AST and alkaline phosphatase levels, in patients who were receiving ATDs [87].
The commercial (Eurosil 85®) formulation of silymarin significantly improved the signs and symptoms of hepatotoxicity in a real-world observational German study that assessed its effect on liver function and quality of life patients with possible DILI (n = 190) [92]. Quality of life was rated by patients on a 6-point Likert scale from 1 to 6 (corresponding with slightly impaired to very strongly impaired). Liver enzyme levels were significantly reduced after ≥ 2 months’ silymarin treatment; liver-related symptoms and quality of life also improved (Fig. 6) [92].

Reproduced with permission from Gillessen et al. [92]
Changes in liver enzymes, liver-related symptoms and quality of life in patients with non-alcoholic liver disease receiving silymarin for 4 months [92]. a Liver function parameters (all comparisons P < 0.001 baseline vs. 4 months); b signs and symptoms of liver disease; c: quality of life. ALT alanine aminotransferase, AP alkaline phosphatase, AST aspartate aminotransferase, GGT gamma-glutamyl transferase, TBIL total bilirubin.
Agents used for cancer chemotherapy are very frequently associated with DILI. For this reason, patients who receive chemotherapy require careful liver function assessment prior to treatment to determine the most appropriate choice of chemotherapeutic agent and whether dose modification is required [93]. In a study in China, patients with acute lymphoblastic or acute myeloid leukemia undergoing chemotherapy (n = 70) received either the Eurosil 85®-derived formulation of silymarin (420 mg/day) plus diammonium glycyrrhizinate or diammonium glycyrrhizinate alone [94]. A greater proportion of silymarin recipients had either no DILI or mild DILI than those who had not received silymarin (measured according to the 1990 Paris International Consensus Conference classification of liver injury) (Fig. 7) [94]. Prevention [95] and treatment [96] of chemotherapy-induced liver disease using silymarin-based formulations were also evaluated in two pilot studies in pediatric patients with acute lymphoblastic leukemia (n = 50 [96], n = 80 [95]). Positive results were obtained with silymarin treatment for improvement in liver enzyme profiles [95, 96], providing preliminary evidence that, despite study limitations, silymarin may be a safe and effective supportive-care agent in patients receiving chemotherapy [96].
Preventive effect on DILI by treatment with the combination of silymarin (420 mg/day) plus diammonium glycyrrhizinate compared with diammonium glycyrrhizinate alone in patients with acute lymphoblastic or acute myeloid leukemia undergoing chemotherapy [94]. DILI was measured according to the 1990 Paris International Consensus Conference classification of liver injury. Chemo chemotherapy, DILI drug-induced liver injury, Glyc diammonium glycyrrhizinate, Sil silymarin
Viral Hepatitis
Because there are safe and effective direct antiviral treatments available, the use of silymarin for this indication has not been extensively investigated. Nevertheless, studies suggest silymarin may have a role as supportive treatment for patients with acute or chronic hepatitis [97,98,99]. It should be noted that silymarin is approved for liver support, not for treatment of viral hepatitis.
In a pre-planned analysis, data on baseline silymarin use were collected for patients enrolled in the large HALT-C (Hepatitis C Antiviral Long-Term Treatment against Cirrhosis) trial; the primary aim of the trial was to assess the long-term use of peginterferon alpha-2a in hepatitis C patients with advanced fibrosis or cirrhosis in whom standard care had failed [97]. Patients were assessed for disease progression (defined as a ≥ 2-point increase in Ishak fibrosis score) at 1.5 and 3.5 year biopsies and followed up for > 8.65 years for clinical outcomes. At baseline, 17% of the 1049 patients had formerly used silymarin (median treatment duration 6 months), and 16% were still using silymarin (median duration 35 months). Although silymarin use had no effect on clinical outcomes, baseline use/former use was significantly associated with less histologic liver disease progression, and current use of silymarin at baseline was also associated with a significantly lower hepatic collagen content in biopsies [97]. In another trial in patients with chronic hepatitis C, 177 Egyptian patients received either the commercial (Eurosil 85®) formulation of silymarin (125 mg) or a low-dose multivitamin supplement three times a day [99]. At a 12-month follow-up, patients from both groups had significant (P < 0.05) improvements from baseline in symptoms of fatigue and weight loss, and silymarin users also had significant improvements in vomiting/heart burn. Although not significant, complaints of jaundice and dark urine decreased from 5.8 to 7.4%, respectively, at baseline to 0% and 1.4% at 12 months in silymarin recipients. Patients in both groups had significant improvements from baseline in almost all quality of life (QOL) scores (assessed using the 36-item short-form health survey, modified to include parameters specific to chronic liver disease), with the exceptions being the social functioning score in the silymarin group and the role emotional score in the multivitamin group. As symptom and QOL improvements were observed in both groups, it is possible that they were related to improved health care (regular nurse home visits and medical care) in a community that generally has limited access to these services [99]. The commercial (Eurosil 85®) formulation of silymarin (140 mg/three times a day) has also been assessed in an 8-week randomized, placebo-controlled trial in Egyptian patients with symptoms of acute viral hepatitis (including ALT levels > 2.5 the upper limit of normal) [98]. Compared with placebo recipients, those receiving silymarin had significantly faster resolution of biliary retention symptoms of dark urine, jaundice and scleral icterus as well as a significant reduction in indirect bilirubin at day 56; there were no significant between-group differences in changes in other indicators of hepatocellular damage [98].
Toxicity and Safety
In clinical trials, silymarin has been used for up to 4 years at doses of up to 420 mg/day (recommended dose) and for up to 48 weeks at 2100 mg/day [30]. Overall, silymarin and silibinin are well tolerated with only minor adverse events reported [13]. Results of systematic reviews of clinical trials of silymarin show a low incidence of adverse events (< 4%, slightly lower than with placebo) and no treatment-related serious adverse events [13, 65, 100] or deaths [13, 100]. In placebo-controlled trials in a total of almost 600 patients, the proportion of patients discontinuing treatment because of adverse events was very low (0.68%) and similar to placebo (0.67%); the most commonly reported (≥ 1% of patients) adverse events in these trials were headaches and pruritus, both of which occurred in < 1.5% of patients [100]. In open-label trials in a total of > 3500 patients, gastrointestinal adverse events (diarrhea, dyspepsia, irregular stools and nausea) were among the most commonly reported; however, all occurred in < 0.25% of patients [100].
In a randomized, phase I dose ascending trial, 32 patients with non-cirrhotic chronic hepatitis C received placebo, the recommended dose of the commercial (Eurosil 85®) formulation of silymarin (140 mg) or one of three higher doses (280 mg, 560 mg or 700 mg) every 8 h for 7 days [23]. Of the 24 patients who received silymarin, 1 in the 240 mg group reported adverse events (mild-to-moderate nausea and headache, both of which resolved within 24 h and were judged to be unrelated to treatment). In another randomized trial, 177 Egyptian patients with chronic hepatitis C received either the commercial (Eurosil 85®) formulation of silymarin (125 mg) or a low-dose multivitamin supplement three times a day [99]. Twelve-month follow-up data were available for 141 patients. The most commonly reported adverse events (> 1 event per person week) were abdominal colic/discomfort (3.6 events per person week for silymarin vs. 3.2 for multivitamins), fatigue (3.5 vs. 4.4), headache (3.3 vs. 3.8) and diarrhea (1.4 vs. 1.7 events per person week). No patients in either group discontinued treatment because of adverse events [99].
In case reports of adverse events, there have been no deaths and only one serious adverse event considered probably related to silymarin [100]. In this case, a 57-year-old woman required hospitalization after experiencing intermittent sweating, nausea, colicky pain, diarrhea, vomiting, weakness and collapse [100]. Mild laxative effects have also been reported in patients taking milk thistle preparations [30, 101]. There have been rare reports of anaphylactic reactions in patients taking milk thistle, one in a patient receiving a standardized preparation [102] and one in an individual who ingested a tea prepared from Fructus Silybi Mariae (non-standardized preparation) [101]. Thus, caution is advised in patients with a known sensitivity to plants in the Asteraceae/Compositae family (members of this family include chrysanthemums, daisies, marigolds and ragweed) [30, 102].
Conclusions
Silymarin has shown positive effects as supportive treatment in most forms of liver disease including cirrhosis and liver damage due to alcohol abuse. In clinical trials that included patients with cirrhosis, there was a significant reduction of liver-related deaths with silymarin treatment [13]. The mechanism of action by which silymarin produces these clinical effects is attributed to its antioxidant activity. It exerts an antioxidant effect by acting as a scavenger of the free radicals that induce lipid peroxidation as well as influencing the enzyme systems associated with the cellular damage that leads to fibrosis and cirrhosis.
By reducing oxidative stress and the consequent cytotoxicity, silymarin protects intact liver cells or cells not yet irreversibly damaged and thus may be considered hepatoprotective. This effect was evident in a study of diabetic patients with mild cirrhosis, in which silymarin reduced signs of hepatic dysfunction and improved glycemic control. Therefore, while silymarin can support liver functionality, even in the more advanced stages of fatty liver disease, for maximum benefit, treatment with silymarin should be initiated as early as possible in patients with fatty liver disease (AFLD or NAFLD) or DILI when the regenerative potential of the liver is still high and when removal of oxidative stress, the cause of cytotoxicity, can achieve the best results.
References
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. https://doi.org/10.1016/j.jhep.2018.09.014.
Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23(4):749–54. https://doi.org/10.1053/jhep.1996.v23.pm0008666328.
Kwon DY, Jung YS, Kim SJ, Kim YS, Choi DW, Kim YC. Alterations in sulfur amino acid metabolism in mice treated with silymarin: a novel mechanism of its action involved in enhancement of the antioxidant defense in liver. Planta Med. 2013;79(12):997–1002. https://doi.org/10.1055/s-0032-1328704.
Song Z, Deaciuc I, Song M, Lee DY, Liu Y, Ji X, McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin Exp Res. 2006;30(3):407–13. https://doi.org/10.1111/j.1530-0277.2006.00063.x.
Valenzuela A, Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol Res. 1994;27(2):105–12.
Valenzuela A, Guerra R. Differential effect of silybin on the Fe2+-ADP and t-butyl hydroperoxide-induced microsomal lipid peroxidation. Experientia. 1986;42(2):139–41.
van Pelt JF, Verslype C, Crabbe T, Zaman Z, Fevery J. Primary human hepatocytes are protected against prolonged and repeated exposure to ethanol by silibinin-dihemisuccinate. Alcohol Alcohol. 2003;38(5):411–4.
Siegel AB, Stebbing J. Milk thistle: early seeds of potential. Lancet Oncol. 2013;14(10):929–30. https://doi.org/10.1016/S1470-2045(13),70414-5.
Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93(2):139–43. https://doi.org/10.1111/j.1572-0241.1998.00139.x.
Hahn G, Lehmann HD, Kurten M, Uebel H, Vogel G. On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) Gaertn. Arzneimittelforschung. 1968;18(6):698–704.
Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev. 2011;16(3):239–49.
Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants (Basel). 2015;4(1):204–47. https://doi.org/10.3390/antiox4010204.
Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–63. https://doi.org/10.2165/00003495-200161140-00003.
Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytother Res. 2010;24(10):1423–32. https://doi.org/10.1002/ptr.3207.
Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124(5):491–504.
Kren V, Walterova D. Silybin and silymarin–new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):29–41.
Wu JW, Lin LC, Hung SC, Lin CH, Chi CW, Tsai TH. Hepatobiliary excretion of silibinin in normal and liver cirrhotic rats. Drug Metab Dispos. 2008;36(3):589–96. https://doi.org/10.1124/dmd.107.017004.
Zhu HJ, Brinda BJ, Chavin KD, Bernstein HJ, Patrick KS, Markowitz JS. An assessment of pharmacokinetics and antioxidant activity of free silymarin flavonolignans in healthy volunteers: a dose escalation study. Drug Metab Dispos. 2013;41(9):1679–85. https://doi.org/10.1124/dmd.113.052423.
Abenavoli L, Aviello G, Capasso R, Milic N, Capasso F. Milk thistle for treatment of nonalcoholic fatty liver disease. Hepat Mon. 2011;11(3):173–7.
Schulz HU, Schurer M, Krumbiegel G, Wachter W, Weyhenmeyer R, Seidel G. The solubility and bioequivalence of silymarin preparations. Arzneimittelforschung. 1995;45(1):61–4.
Wachter W, Zaeske H, inventors. Process for the manufacture of flavanolignan preparations with improved release and absorbability, compositions obtainable thereby and their use for the preparation of pharmaceuticals. United States patent US 5906991. 1999 May 25.
Wachter W, Zaeske H, inventors. Process for the preparation of flavano lignan preparations with improved release and absorbability thereafter available preparations and their use for the manufacture of medicines. Germany patent DE 19501266. 1996 July 25.
Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, Berman J, Liu QY, Doo E, Fried MW. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50(4):434–49. https://doi.org/10.1177/0091270009347475.
Flory PJ, Krug G, Lorenz D, Mennicke WH. Studies on elimination of silymarin in cholecystectomized patients. I. Biliary and renal elimination after a single oral dose. Planta Med. 1980;38(3):227–37. https://doi.org/10.1055/s-2008-1074867.
Lorenz D, Lucker PW, Mennicke WH, Wetzelsberger N. Pharmacokinetic studies with silymarin in human serum and bile. Methods Find Exp Clin Pharmacol. 1984;6(10):655–61.
Morazzoni P, Montalbetti A, Malandrino S, Pifferi G. Comparative pharmacokinetics of silipide and silymarin in rats. Eur J Drug Metab Pharmacokinet. 1993;18(3):289–97. https://doi.org/10.1007/BF03188811.
Lorenz D, Mennicke WH, Behrendt W. Elimination of silymarin by cholecystectomied patients. 2. Biliary elimination after multiple oral doses. Planta Med. 1982;45(4):216–23.
Schandalik R, Gatti G, Perucca E. Pharmacokinetics of silybin in bile following administration of silipide and silymarin in cholecystectomy patients. Arzneimittelforschung. 1992;42(7):964–8.
Doehmer J, Tewes B, Klein KU, Gritzko K, Muschick H, Mengs U. Assessment of drug–drug interaction for silymarin. Toxicol In Vitro. 2008;22(3):610–7. https://doi.org/10.1016/j.tiv.2007.11.020.
Natural Medicines Comprehensive Database. Milk Thistle. 2018. http://www.naturaldatabase.com/.
Teschke R. Alcoholic liver disease: alcohol metabolism, cascade of molecular mechanisms, cellular targets, and clinical aspects. Biomedicines. 2018. https://doi.org/10.3390/biomedicines6040106.
Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–94. https://doi.org/10.1038/nri3623.
Dehmlow C, Murawski N, de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58(18):1591–600.
Valenzuela A, Guerra R, Garrido A. Silybin dihemisuccinate protects rat erythrocytes against phenylhydrazine-induced lipid peroxidation and hemolysis. Planta Med. 1987;53(5):402–5. https://doi.org/10.1055/s-2006-962757.
Noel-Hudson MS, de Belilovsky C, Petit N, Lindenbaum A, Wepierre J. In vitro cytotoxic effects of enzymatically induced oxygen radicals in human fibroblasts: experimental procedures and protection by radical scavengers. Toxicol In Vitro. 1989;3(2):103–9.
Li CC, Hsiang CY, Wu SL, Ho TY. Identification of novel mechanisms of silymarin on the carbon tetrachloride-induced liver fibrosis in mice by nuclear factor-kappaB bioluminescent imaging-guided transcriptomic analysis. Food Chem Toxicol. 2012;50(5):1568–75. https://doi.org/10.1016/j.fct.2012.02.025.
Gharagozloo M, Velardi E, Bruscoli S, Agostini M, Di Sante M, Donato V, Amirghofran Z, Riccardi C. Silymarin suppress CD4+ T cell activation and proliferation: effects on NF-kappaB activity and IL-2 production. Pharmacol Res. 2010;61(5):405–9. https://doi.org/10.1016/j.phrs.2009.12.017.
Trappoliere M, Caligiuri A, Schmid M, Bertolani C, Failli P, Vizzutti F, Novo E, di Manzano C, Marra F, Loguercio C, Pinzani M. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009;50(6):1102–11. https://doi.org/10.1016/j.jhep.2009.02.023.
Faulstich H, Jahn W, Wieland T. Silybin inhibition of amatoxin uptake in the perfused rat liver. Arzneimittelforschung. 1980;30(3):452–4.
El-Bahay C, Gerber E, Horbach M, Tran-Thi QH, Rohrdanz E, Kahl R. Influence of tumor necrosis factor-alpha and silibin on the cytotoxic action of alpha-amanitin in rat hepatocyte culture. Toxicol Appl Pharmacol. 1999;158(3):253–60. https://doi.org/10.1006/taap.1999.8705.
Saller R, Melzer J, Reichling J, Brignoli R, Meier R. An updated systematic review of the pharmacology of silymarin. Forsch Komplementmed. 2007;14(2):70–80. https://doi.org/10.1159/000100581.
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. https://doi.org/10.1002/hep.28431.
Kondylis V, Kumari S, Vlantis K, Pasparakis M. The interplay of IKK, NF-kappaB and RIPK1 signaling in the regulation of cell death, tissue homeostasis and inflammation. Immunol Rev. 2017;277(1):113–27. https://doi.org/10.1111/imr.12550.
Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in cAMP primes macrophages to enhanced LPS-inducible NF-kappaB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006;291(4):G681–8. https://doi.org/10.1152/ajpgi.00098.2006.
Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16(1):203. https://doi.org/10.1186/s12944-017-0572-9.
Guan YS, He Q, Wang MQ, Li P. Nuclear factor kappa B and hepatitis viruses. Expert Opin Ther Targets. 2008;12(3):265–80. https://doi.org/10.1517/14728222.12.3.265.
Muriel P. NF-kappaB in liver diseases: a target for drug therapy. J Appl Toxicol. 2009;29(2):91–100. https://doi.org/10.1002/jat.1393.
Robinson SM, Mann DA. Role of nuclear factor kappaB in liver health and disease. Clin Sci (Lond). 2010;118(12):691–705. https://doi.org/10.1042/CS20090549.
Lieber CS, Leo MA, Cao Q, Ren C, DeCarli LM. Silymarin retards the progression of alcohol-induced hepatic fibrosis in baboons. J Clin Gastroenterol. 2003;37(4):336–9.
Wenfeng Z, Yakun W, Di M, Jianping G, Chuanxin W, Chun H. Kupffer cells: increasingly significant role in nonalcoholic fatty liver disease. Ann Hepatol. 2014;13(5):489–95.
Kim M, Yang SG, Kim JM, Lee JW, Kim YS, Lee JI. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: analysis of isolated hepatic stellate cells. Int J Mol Med. 2012;30(3):473–9. https://doi.org/10.3892/ijmm.2012.1029.
Yao J, Zhi M, Gao X, Hu P, Li C, Yang X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz J Med Biol Res. 2013;46(3):270–7.
European Association for the Study of the Liver (EASL). EASL Clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–81. https://doi.org/10.1016/j.jhep.2018.03.018.
O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology. 2010;51(1):307–28. https://doi.org/10.1002/hep.23258.
Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9(1):105–13.
Lucena MI, Andrade RJ, de la Cruz JP, Rodriguez-Mendizabal M, Blanco E, Sanchez de la Cuesta F. Effects of silymarin MZ-80 on oxidative stress in patients with alcoholic cirrhosis. Results of a randomized, double-blind, placebo-controlled clinical study. Int J Clin Pharmacol Ther. 2002;40(1):2–8.
Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, Panes J, Rigau J, Santos J, Rodes J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28(4):615–21.
Trinchet JC, Coste T, Levy VG, Vivet F, Duchatelle V, Legendre C, Gotheil C, Beaugrand M. Treatment of alcoholic hepatitis with silymarin. A double-blind comparative study in 116 patients. Gastroenterol Clin Biol. 1989;13(2):120–4.
Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26(4):871–9.
Bunout D, Hirsch S, Petermann M, de la Maza MP, Silva G, Kelly M, Ugarte G, Iturriaga H. Controlled study of the effect of silymarin on alcoholic liver disease. Rev Med Chil. 1992;120(12):1370–5.
Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand J Gastroenterol. 1982;17(4):517–21.
Feher J, Deak G, Muzes G, Lang I, Niederland V, Nekam K, Karteszi M. Liver-protective action of silymarin therapy in chronic alcoholic liver diseases. Orv Hetil. 1989;130(51):2723–7.
Muzes G, Deak G, Lang I, Nekam K, Niederland V, Feher J. Effect of silimarin (Legalon) therapy on the antioxidant defense mechanism and lipid peroxidation in alcoholic liver disease (double blind protocol). Orv Hetil. 1990;131(16):863–6.
Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85.
Rambaldi A, Jacobs BP, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C virus liver diseases. Cochrane Database Syst Rev. 2007. https://doi.org/10.1002/14651858.cd003620.pub3.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–57. https://doi.org/10.1002/hep.29367.
Hossain N, Kanwar P, Mohanty SR. A comprehensive updated review of pharmaceutical and nonpharmaceutical treatment for NAFLD. Gastroenterol Res Pract. 2016;2016:7109270. https://doi.org/10.1155/2016/7109270.
Wong RJ, Ahmed A. Obesity and non-alcoholic fatty liver disease: disparate associations among Asian populations. World J Hepatol. 2014;6(5):263–73. https://doi.org/10.4254/wjh.v6.i5.263.
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–402. https://doi.org/10.1016/j.jhep.2015.11.004.
Attar BM, Van Thiel DH. Current concepts and management approaches in nonalcoholic fatty liver disease. Sci World J. 2013;2013:481893. https://doi.org/10.1155/2013/481893.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–23. https://doi.org/10.1002/hep.25762.
Centis E, Marzocchi R, Di Domizio S, Ciaravella MF, Marchesini G. The effect of lifestyle changes in non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):267–73. https://doi.org/10.1159/000282101.
Marra F, Lotersztajn S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr Pharm Des. 2013;19(29):5250–69.
Ha HL, Shin HJ, Feitelson MA, Yu DY. Oxidative stress and antioxidants in hepatic pathogenesis. World J Gastroenterol. 2010;16(48):6035–43. https://doi.org/10.3748/wjg.v16.i48.6035.
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16(11):26087–124. https://doi.org/10.3390/ijms161125942.
Ore A, Akinloye OA. Oxidative stress and antioxidant biomarkers in clinical and experimental models of non-alcoholic fatty liver disease. Medicina (Kaunas). 2019. https://doi.org/10.3390/medicina55020026.
Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–53. https://doi.org/10.1016/j.bbagen.2012.09.008.
Lushchak VI. Glutathione homeostasis and functions: potential targets for medical interventions. J Amino Acids. 2012;2012:736837. https://doi.org/10.1155/2012/736837.
Wah Kheong C, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15(12):1940–9. https://doi.org/10.1016/j.cgh.2017.04.016(e1948).
Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nonalcoholic Steatohepatitis Clinical Research N. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. Jama. 2011;305(16):1659–68. https://doi.org/10.1001/jama.2011.520.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, Nash CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. https://doi.org/10.1056/NEJMoa0907929.
Butorova LI, Tsibizova TA, Kalinin AV. Potential for the use of Legalon in non-alcoholic fatty liver disease. Exp Clin Gastroenterol. 2010;3:85–91.
Sorrentino G, Crispino P, Coppola D, De Stefano G. Efficacy of lifestyle changes in subjects with non-alcoholic liver steatosis and metabolic syndrome may be improved with an antioxidant nutraceutical: a controlled clinical study. Drugs R D. 2015;15(1):21–5. https://doi.org/10.1007/s40268-015-0084-x.
Broussard CN, Aggarwal A, Lacey SR, Post AB, Gramlich T, Henderson JM, Younossi ZM. Mushroom poisoning-from diarrhea to liver transplantation. Am J Gastroenterol. 2001;96(11):3195–8. https://doi.org/10.1111/j.1572-0241.2001.05283.x.
French LK, Hendrickson RG, Horowitz BZ. Amanita phalloides poisoning. Clin Toxicol (Phila). 2011;49(2):128–9. https://doi.org/10.3109/15563650.2011.557663.
Mengs U, Pohl RT, Mitchell T. Legalon® SIL: the antidote of choice in patients with acute hepatotoxicity from amatoxin poisoning. Curr Pharm Biotechnol. 2012;13(10):1964–70.
Tao L, Qu X, Zhang Y, Song Y, Zhang SX. Prophylactic therapy of silymarin (milk thistle) on antituberculosis drug-induced liver injury: a meta-analysis of randomized controlled trials. Can J Gastroenterol Hepatol. 2019;2019:3192351. https://doi.org/10.1155/2019/3192351.
Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369(26):2525–34. https://doi.org/10.1056/NEJMra1208937.
Baskaran UL, Sabina EP. Clinical and experimental research in antituberculosis drug-induced hepatotoxicity: a review. J Integr Med. 2017;15(1):27–36. https://doi.org/10.1016/S2095-4964(17),60319-4.
Gu J, Tang SJ, Tan SY, Wu Q, Zhang X, Liu CX, Gao XS, Yuan BD, Han LJ, Gao AP, Wu MY, Huang LH, Ma J, Xiao HP. An open-label, randomized and multi-center clinical trial to evaluate the efficacy of Silibinin in preventing drug-induced liver injury. Int J Clin Exp Med. 2015;8(3):4320–7.
Luangchosiri C, Thakkinstian A, Chitphuk S, Stitchantrakul W, Petraksa S, Sobhonslidsuk A. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015;15:334. https://doi.org/10.1186/s12906-015-0861-7.
Gillessen A, Herrmann WA, Kemper M, Morath H, Mann K. Effect of silymarin on liver health and quality of life. Results of a non-interventional study. MMW Fortschr Med. 2014;156(Suppl 4):120–6.
Vincenzi B, Russo A, Terenzio A, Galvano A, Santini D, Vorini F, Antonelli-Incalzi R, Vespasiani-Gentilucci U, Tonini G. The use of SAMe in chemotherapy-induced liver injury. Crit Rev Oncol Hematol. 2018;130:70–7. https://doi.org/10.1016/j.critrevonc.2018.06.019.
Fengyun C. Silymarin combined with diammonium glycyrrhizinate for prevention and treatment of liver injury induced by chemotherapy in acute leukemia. China Med. 2012;7:902.
Hagag AA, Elgamsy MA, El-Asy HM, Mabrouk MM. Protective role of silymarin on hepatic and renal toxicity induced by MTX based chemotherapy in children with acute lymphoblastic leukemia. Mediterr J Hematol Infect Dis. 2016;8(1):e2016043. https://doi.org/10.4084/MJHID.2016.043.
Ladas EJ, Kroll DJ, Oberlies NH, Cheng B, Ndao DH, Rheingold SR, Kelly KM. A randomized, controlled, double-blind, pilot study of milk thistle for the treatment of hepatotoxicity in childhood acute lymphoblastic leukemia (ALL). Cancer. 2010;116(2):506–13. https://doi.org/10.1002/cncr.24723.
Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE, Group H-CT. Silymarin use and liver disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Aliment Pharmacol Ther. 2011;33(1):127–37. https://doi.org/10.1111/j.1365-2036.20.
El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, Hashem M, Mousa A, Aboul-Fotouh A, El-Kassas M, Esmat G, Strickland GT. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine. 2009;16(5):391–400. https://doi.org/10.1016/j.phymed.2009.02.002.
Tanamly MD, Tadros F, Labeeb S, Makld H, Shehata M, Mikhail N, Abdel-Hamid M, Shehata M, Abu-Baki L, Medhat A, Magder LS, Afdhal NH, Strickland GT. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36(11):752–9. https://doi.org/10.1016/j.dld.2004.06.015.
Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15(1):9–20. https://doi.org/10.1159/000113648.
World Health Organization. Fructus silybi mariae. In: WHO monographs on selected medicinal plants, vol 2; 2004. https://apps.who.int/medicinedocs/en/d/Js4927e/.
Mills SY, Simon Mills MFMA, Bone K. The essential guide to herbal safety: Elsevier Health Sciences; 2004. https://www.elsevier.com/books/the-essential-guide-to-herbal-safety/mills/978-0-443-07171-3.
Acknowledgements
Funding
This manuscript, including the journal’s Rapid Service and Open Access Fees, was supported by Meda Pharma SpA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Authorship Contributions
All authors contributed equally to this paper related to the conception, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
Editorial Assistance
Editorial assistance for editing this article was provided by Catherine Rees of Springer Healthcare Communications. Support for this assistance was funded by Meda Pharma SpA.
Disclosures
Anton Gillessen and Hartmut H-J Schmidt declare no conflicts of interest for the present work. Anton Gillessen has received research grants,and speaker honoraria from Abbvie, Falk, Gilead, Intercept, Leo, Meda, Merck, MSD, Norgine, Novartis, Olympus, Schwabe and UCB, unrelated to the present work.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11778396.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gillessen, A., Schmidt, H.HJ. Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv Ther 37, 1279–1301 (2020). https://doi.org/10.1007/s12325-020-01251-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01251-y