Abstract
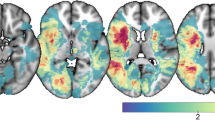
Microstructural changes after an ischemic stroke (IS) have mainly been described in white matter. Data evaluating microstructural changes in gray matter (GM) remain scarce. The aim of the present study was to evaluate the integrity of GM on longitudinal data using mean diffusivity (MD), and its influence on post-IS cognitive performances. A prospective study was conducted, including supra-tentorial IS patients without pre-stroke disability. A cognitive assessment was performed at baseline and 1 year, including a Montreal Cognitive Assessment, an Isaacs set test, and a Zazzo cancelation task (ZCT): completion time and number of errors. A 3-T brain MRI was performed at the same two time-points, including diffusion tensor imaging for the assessment of GM MD. GM volume was also computed, and changes in GM volume and GM MD were evaluated, followed by the assessment of the relationship between these structural changes and changes in cognitive performances. One hundred and four patients were included (age 68.5 ± 21.5, 38.5% female). While no GM volume loss was observed, GM MD increased between baseline and 1 year. The increase of GM MD in left fronto-temporal regions (dorsolateral prefrontal cortex, superior and medial temporal gyrus, p < 0.05, Threshold-Free Cluster Enhancement, 5000 permutations) was associated with an increase time to complete ZCT, regardless of demographic confounders, IS volume and location, GM, and white matter hyperintensity volume. GM integrity deterioration was thus associated with processing speed slowdown, and appears to be a biomarker of cognitive frailty. This broadens the knowledge of post-IS cognitive impairment mechanisms.


Similar content being viewed by others
Abbreviations
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion weighted imaging
- ET:
-
Echo time
- FLAIR:
-
Fluid-attenuated inversion recovery
- FOV:
-
Field of view
- FSL:
-
FMRIB software library
- FEW:
-
Family-wise error
- GM:
-
Gray matter
- IQCODE:
-
Informant Questionnaire in Cognitive Decline in the Elderly
- IS:
-
Ischemic stroke
- IST:
-
Isaacs set test
- MD:
-
Mean diffusivity
- MNI:
-
Montreal Neurological Institute
- MoCA:
-
Montreal Cognitive Assessment
- NIHSS:
-
National Institute of Health Stroke Score
- RT:
-
Repetition time
- SPM:
-
Statistical parametric mapping
- TFCE:
-
Threshold-free cluster enhancement
- TIV:
-
Total intracranial volume
- WMH:
-
White matter hyperintensities
- ZCT:
-
Zazzo’s cancelation task
References
Pendlebury ST. Dementia in patients hospitalized with stroke: rates, time course, and clinico-pathologic factors. Int J Stroke. 2012;7:570–81.
Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–18.
Kliper E, Ben Assayag E, Tarrasch R, Artzi M, Korczyn AD, Shenhar-Tsarfaty S, et al. Cognitive state following stroke: the predominant role of preexisting white matter lesions. PloS One. 2014;9:e105461.
Sagnier S, Catheline G, Dilharreguy B, Linck P-A, Coupé P, Munsch F, et al. Normal-appearing white matter integrity is a predictor of outcome after ischemic stroke. Stroke. 2020;51:449–56.
Salminen LE, Conturo TE, Laidlaw DH, Cabeen RP, Akbudak E, Lane EM, et al. Regional age differences in gray matter diffusivity among healthy older adults. Brain Imaging Behav. 2016;10:203–11.
Weston PSJ, Simpson IJA, Ryan NS, Ourselin S, Fox NC. Diffusion imaging changes in grey matter in Alzheimer’s disease: a potential marker of early neurodegeneration. Alzheimers Res Ther. 2015;7:47.
Molko N, Pappata S, Mangin JF, Poupon C, Vahedi K, Jobert A, et al. Diffusion tensor imaging study of subcortical gray matter in CADASIL. Stroke. 2001;32:2049–54.
O’Sullivan M, Singhal S, Charlton R, Markus HS. Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology. 2004;62:702–7.
Quintaine V, Chabriat H, Jouvent E, Yelnik A. MRI ameliorates the prediction of further clinical evolution even months after ischemic stroke. Ann Phys Rehabil Med. 2015;58:e6.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38.
Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21.
Avants BB, Tustison N, Johnson H. Advanced normalization tools (ANTS). Release 1.5. Penn Image Computing and Science Laboratory. University of Pennsylvania. 2011.
Preziosa P, Kiljan S, Steenwijk MD, Meani A, van de Berg WDJ, Schenk GJ, et al. Axonal degeneration as substrate of fractional anisotropy abnormalities in multiple sclerosis cortex. Brain. 2019;142:1921–37.
Etherton MR, Wu O, Cougo P, Giese A-K, Cloonan L, Fitzpatrick KM, et al. Integrity of normal-appearing white matter and functional outcomes after acute ischemic stroke. Neurology. 2017;88:1701–8.
Etherton MR, Wu O, Giese A-K, Lauer A, Boulouis G, Mills B, et al. White matter integrity and early outcomes after acute ischemic stroke. Transl Stroke Res. 2019;10:630–8.
Rasquin SMC, Lodder J, Ponds RWHM, Winkens I, Jolles J, Verhey FRJ. Cognitive functioning after stroke: a one-year follow-up study. Dement Geriatr Cogn Disord. 2004;18:138–44.
Hochstenbach J, Mulder T, van Limbeek J, Donders R, Schoonderwaldt H. Cognitive decline following stroke: a comprehensive study of cognitive decline following stroke. J Clin Exp Neuropsychol. 1998;20:503–17.
Meijer KA, van Geest Q, Eijlers AJC, Geurts JJG, Schoonheim MM, Hulst HE. Is impaired information processing speed a matter of structural or functional damage in MS? NeuroImage Clin. 2018;20:844–50.
Wright SN, Hong LE, Winkler AM, Chiappelli J, Nugent K, Muellerklein F, et al. Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum Brain Mapp. 2015;36:3793–804.
Munsch F, Sagnier S, Asselineau J, Bigourdan A, Guttmann CR, Debruxelles S, et al. Stroke location is an independent predictor of cognitive outcome. Stroke. 2016;47:66–73.
Ernst M, Boers AMM, Forkert ND, Berkhemer OA, Roos YB, Dippel DWJ, et al. Impact of ischemic lesion location on the mRS score in patients with ischemic stroke: a voxel-based approach. Am J Neuroradiol. 2018;39:1989–94.
Zhang J, Zhang Y, Xing S, Liang Z, Zeng J. Secondary neurodegeneration in remote regions after focal cerebral infarction. Stroke. 2012;43:1700–5.
Kuchcinski G, Munsch F, Lopes R, Bigourdan A, Su J, Sagnier S, et al. Thalamic alterations remote to infarct appear as focal iron accumulation and impact clinical outcome. Brain. 2017;140:1932–46.
Vrenken H, Pouwels PJW, Geurts JJG, Knol DL, Polman CH, Barkhof F, et al. Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J Magn Reson Imaging. 2006;23:628–36.
Wang Z, Zhang S, Liu C, Yao Y, Shi J, Zhang J, et al. A study of neurite orientation dispersion and density imaging in ischemic stroke. Magn Reson Imaging. 2019;57:28–33.
Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66:814–23.
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
Isaacs B, Kennie AT. The set test as an aid to the detection of dementia in old people. Br J Psychiatry J Ment Sci. 1973;123:467–70.
Zazzo R. Manuel pour l’examen psychologique de l’enfant. Neuchâtel: Delachaux et Niestlé; 1969.
Jorm AF. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): a review. Int Psychogeriatr IPA. 2004;16:275–93.
Funding
The study was supported by public grants (PHRCI-2012 and ANR-10-LABX-57 from the Translational Research and Advanced Imaging Laboratory).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Regional French Human Protection Committee (CPP 2012/19 2012-A00190-43).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sagnier, S., Catheline, G., Dilharreguy, B. et al. Microstructural Gray Matter Integrity Deteriorates After an Ischemic Stroke and Is Associated with Processing Speed. Transl. Stroke Res. 14, 185–192 (2023). https://doi.org/10.1007/s12975-022-01020-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01020-9