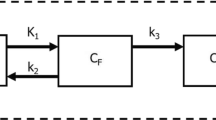
Abstract
Gliomas are the most common type of primary brain tumors and are classified as grade IV. Necrosis and hypoxia are essential diagnostic features which result in poor prognosis of gliomas. The aim of this study was to report quantitative temporal analyses aiming at determining the hypoxic regions in glioblastoma multiforme and to suggest an optimal time for the clinical single scan of hypoxia. Nine subjects were imaged with PET and 18F-FMISO in dynamic mode for 15 min followed with static scans at 2, 3 and 4 h post-injection. Spectral analysis, tumor-to-blood ratio (TBR) and tumor-to-normal tissue ratio (TNR) were used to delimit perfused and hypoxic tumor regions. TBR and TNR images were further scaled by thresholding at 1.2, 1.4, 2 and 2.5 levels. The images showed a varying tumor volume with time. TBR produced broader images of the tumor than TNR considering the same thresholds on intensity. Spectral analysis reliably determined hypoxia with different degrees of perfusion. By comparing TBR and TNR with spectral analysis images, weak to moderate correlation coefficients were found for most thresholding values and imaging times (range: 0 to 0.69). Hypoxic volume (HV) estimated from the net uptake rate (Ki) were changing among imaging times. The minimum HV changes were found between 3 h and 4 h, confirming that after 3 h, there was a very low exchange of 81F-FMISO between blood and tumor. On the other hand, hypoxia started to dominate the perfused tissue at 90 min, suggesting this time is suitable for a single scan acquisition irrespective of tumor status being highly hypoxic or perfused. At this time, TBR and TNR were respectively found in the nine subjects as 1.72 ± 0.22 and 1.74 ± 0.19.








Similar content being viewed by others
References
Gilbert MR (2011) Recurrent glioblastoma: a fresh look at current therapies and emerging novel approaches. Semin Oncol 38(Suppl 4):S21–33
Hirata K, Terasaka S, Shiga T et al (2012) (1)(8)F-Fluoromisonidazole positron emission tomography may differentiate glioblastoma multiforme from less malignant gliomas. Eur J Nucl Med Mol Imaging 39:760–770
Zygogianni A, Protopapa M, Kougioumtzopoulou A et al (2018) From imaging to biology of glioblastoma: new clinical oncology perspectives to the problem of local recurrence. Clin Transl Oncol 20:989–1003
Bekaert L, Valable S, Lechapt-Zalcman E et al (2017) [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging 44:1383–1392
Thorwarth D, Eschmann SM, Paulsen F et al (2005) A kinetic model for dynamic [18F]-Fmiso PET data to analyse tumour hypoxia. Phys Med Biol 50:2209–2224
Zimny M, Gagel B, DiMartino E et al (2006) FDG–a marker of tumour hypoxia? A comparison with [18F]fluoromisonidazole and pO2-polarography in metastatic head and neck cancer. Eur J Nucl Med Mol Imaging 33:1426–1431
Toyonaga T, Yamaguchi S, Hirata K et al (2017) Hypoxic glucose metabolism in glioblastoma as a potential prognostic factor. Eur J Nucl Med Mol Imaging 44:611–619
Toyonaga T, Hirata K, Yamaguchi S et al (2016) (18)F-fluoromisonidazole positron emission tomography can predict pathological necrosis of brain tumors. Eur J Nucl Med Mol Imaging 43:1469–1476
Thorwarth D, Eschmann SM, Holzner F et al (2006) Combined uptake of [18F]FDG and [18F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol 80:151–156
Eschmann SM, Paulsen F, Bedeshem C et al (2007) Hypoxia-imaging with (18)F-Misonidazole and PET: changes of kinetics during radiotherapy of head-and-neck cancer. Radiother Oncol 83:406–410
Yamamoto Y, Maeda Y, Kawai N et al (2012) Hypoxia assessed by 18F-fluoromisonidazole positron emission tomography in newly diagnosed gliomas. Nucl Med Commun 33:621–625
Kawai N, Maeda Y, Kudomi N et al (2011) Correlation of biological aggressiveness assessed by 11C-methionine PET and hypoxic burden assessed by 18F-fluoromisonidazole PET in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging 38:441–450
Swanson KR, Chakraborty G, Wang CH et al (2009) Complementary but distinct roles for MRI and 18F-fluoromisonidazole PET in the assessment of human glioblastomas. J Nucl Med 50:36–44
Casciari JJ, Graham MM, Rasey JS (1995) A modeling approach for quantifying tumor hypoxia with [F-18]fluoromisonidazole PET time-activity data. Med Phys 22:1127–1139
Wang W, Georgi JC, Nehmeh SA et al (2009) Evaluation of a compartmental model for estimating tumor hypoxia via FMISO dynamic PET imaging. Phys Med Biol 54:3083–3099
Bruehlmeier M, Roelcke U, Schubiger PA et al (2004) Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O–H2O. J Nucl Med 45:1851–1859
Koch CJ, Evans SM (2015) Optimizing hypoxia detection and treatment strategies. Semin Nucl Med 45:163–176
Pajonk F, Vlashi E, McBride WH (2010) Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells 28:639–648
Preibisch C, Shi K, Kluge A et al (2017) Characterizing hypoxia in human glioma: a simultaneous multimodal MRI and PET study. NMR Biomed 30:e3775
Grkovski M, Schoder H, Lee NY et al (2017) Multiparametric imaging of tumor hypoxia and perfusion with (18)F-fluoromisonidazole dynamic PET in head and neck cancer. J Nucl Med 58:1072–1080
Taylor E, Gottwald J, Yeung I et al (2017) Impact of tissue transport on PET hypoxia quantification in pancreatic tumours. EJNMMI Res 7:101
Veronese M, Rizzo G, Bertoldo A et al (2016) Spectral analysis of dynamic PET studies: a review of 20 years of method developments and applications. Comput Math Methods Med 2016:7187541
Bentourkia M (2003) PET kinetic modeling of 11C-acetate from projections. Comput Med Imaging Graph 27:373–379
Murase K, Inoue T, Fujioka H et al (1999) An alternative approach to estimation of the brain perfusion index for measurement of cerebral blood flow using technetium-99m compounds. Eur J Nucl Med 26:1333–1339
Cunningham VJ, Jones T (1993) Spectral analysis of dynamic PET studies. J Cereb Blood Flow Metab 13:15–23
Bertoldo A, Vicini P, Sambuceti G et al (1998) Evaluation of compartmental and spectral analysis models of [18F]FDG kinetics for heart and brain studies with PET. IEEE Trans Biomed Eng 45:1429–1448
Meikle SR, Matthews JC, Cunningham VJ et al (1998) Parametric image reconstruction using spectral analysis of PET projection data. Phys Med Biol 43:651–666
Turkheimer F, Sokoloff L, Bertoldo A et al (1998) Estimation of component and parameter distributions in spectral analysis. J Cereb Blood Flow Metab 18:1211–1222
de Geus-Oei LF, Visser EP, Krabbe PF et al (2006) Comparison of image-derived and arterial input functions for estimating the rate of glucose metabolism in therapy-monitoring 18F-FDG PET studies. J Nucl Med 47:945–949
Grecchi E, Veronese M, Moresco RM et al (2016) Quantification of dynamic [18F]FDG pet studies in acute lung injury. Mol Imaging Biol 18:143–152
Silvestri E, Scolozzi V, Rizzo G et al (2018) The kinetics of (18)F-FDG in lung cancer: compartmental models and voxel analysis. EJNMMI Res 8:88
Boutchko R, Mitra D, Baker SL et al (2015) Clustering-initiated factor analysis application for tissue classification in dynamic brain positron emission tomography. J Cereb Blood Flow Metab 35:1104–1111
Wardak M, Schiepers C, Dahlbom M et al (2011) Discriminant analysis of (1)(8)F-fluorothymidine kinetic parameters to predict survival in patients with recurrent high-grade glioma. Clin Cancer Res 17:6553–6562
Bentourkia M, Bol A, Ivanoiu A et al (1999) A standardized blood sampling scheme in quantitative FDG-PET studies. IEEE Trans Med Imaging 18:379–384
Kemp BJ, Kim C, Williams JJ et al (2006) NEMA NU 2–2001 performance measurements of an LYSO-based PET/CT system in 2D and 3D acquisition modes. J Nucl Med 47:1960–1967
Lim J-L, Berridge MS (1993) An efficient radiosynthesis of [18F]fluoromisonidazole. Appl Radiat Isot 44:1085–1091
Turkheimer F, Moresco RM, Lucignani G et al (1994) The use of spectral analysis to determine regional cerebral glucose utilization with positron emission tomography and [18F]fluorodeoxyglucose: theory, implementation, and optimization procedures. J Cereb Blood Flow Metab 14:406–422
Gunn RN, Gunn SR, Cunningham VJ (2001) Positron emission tomography compartmental models. J Cereb Blood Flow Metab 21:635–652
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdo, Ra., Lamare, F., Fernandez, P. et al. Analysis of hypoxia in human glioblastoma tumors with dynamic 18F-FMISO PET imaging. Australas Phys Eng Sci Med 42, 981–993 (2019). https://doi.org/10.1007/s13246-019-00797-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-019-00797-8