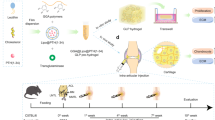
Abstract
Intra-articular (IA) administration of drugs is an appealing route for the effective treatment of large-joint diseases. However, a key limitation of this route is the premature elimination of the injected drugs from the synovial cavity. The objective of this work was to develop an easily injectable controlled release system intended to prolong the activity of anti-inflammatory drugs in the articular cavity. The system was an in situ forming hydrogel, made of fibrin and hyaluronic acid (HA), loaded with nanocapsules (NCs). The NCs, consisting of an olive oil core surrounded by a HA shell, were loaded with two different drugs, dexamethasone (DMX) and a galectin-3 inhibitor. They presented a particle size in the range of 122–135 nm and a surface charge of − 29/− 31 mV. The gelation time, rheological properties and porosity of the system could be adjusted by different parameters, such as addition of fibrin crosslinkers factor XIII and α2-antiplasmin. The non-crosslinked HA-fibrin hydrogels containing 30% (v/v) NCs showed the capacity to control the release of the encapsulated drug, DMX, for 72 h in simulated synovial fluid. The preliminary in vivo evaluation of the system containing a galectin-3 inhibitor in an acute synovitis rat model showed a suppression of inflammation after IA administration compared with the non-treated control. In brief, this work shows the possibility to combine an in situ forming hydrogel and NCs as a drug delivery strategy for IA administration and suggests its potential for the treatment of arthropathies.
Lay Summary
This work describes the development and characterization of a new in situ forming hydrogel adapted for intra-articular administration of anti-inflammatory drugs. The prolonged local delivery of these drugs is expected to improve the treatment of large-joint arthropathies. To achieve this objective, the hydrogel, made of biodegradable materials, was loaded with nanodeposits of drugs, named nanocapsules. The efficacy of the system, containing a new galectin-3 inhibitor as a drug candidate, was tested in a rat model of acute synovial inflammation. These results represent the first insights on the in vivo activity of a new galectin-3 inhibitor on a potential galectin-3 immunotherapeutic target for inflammatory joints diseases.

Graphical Abstract





Similar content being viewed by others
References
Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol Nat Publ Group. 2015;10:11–22.
Burt HM, Tsallas A, Gilchrist S, Liang LS. Intra-articular drug delivery systems: overcoming the shortcomings of joint disease therapy. Expert Opin Drug Deliv. 2009;6:17–26.
Kang ML, Im G-I. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin Drug Deliv. 2014;11:269–82.
Wu Q, Wang N, He T, Shang J, Li L, Song L, et al. Thermosensitive hydrogel containing dexamethasone micelles for preventing postsurgical adhesion in a repeated-injury model. Sci Rep. 2015;5:13553.
Ho MJ, Kim SR, Choi YW, Kang MJ. Recent advances in intra-articular drug delivery systems to extend drug retention in joint. J Pharm Investig. 2018;49:9–15.
Van Den Hoven JM, Van Tomme SR, Metselaar JM, Nuijen B, Beijnen JH, Storm G. Liposomal drug formulations in the treatment of rheumatoid arthritis. Mol Pharm. 2011;8:1002–15.
Kapoor B, Singh SK, Gulati M, Gupta R, Vaidya Y. Application of liposomes in treatment of rheumatoid arthritis: quo vadis. Sci World J. 2014;2014.
Sarkar A, Carvalho E, D’Souza AA, Banerjee R. Liposome-encapsulated fish oil protein-tagged gold nanoparticles for intra-articular therapy in osteoarthritis. Nanomedicine. 2019;14:871–87.
Huang G, Zhang Z. Micro- and nano-carrier mediated intra-articular drug delivery systems for the treatment of osteoarthritis. J Nanotechnol. 2012;2012.
Yang M, Feng X, Ding J, Chang F, Chen X. Nanotherapeutics relieve rheumatoid arthritis. J Control Release. 2017;252:108–24.
Bartneck M, Peters FM, Warzecha KT, Bienert M, van Bloois L, Trautwein C, et al. Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages. Nanomed Nanotechnol Biol Med. 2014;10:1209–20.
Hofkens W, Schelbergen R, Storm G, van den Berg WB, van Lent PL. Liposomal targeting of prednisolone phosphate to synovial lining macrophages during experimental arthritis inhibits M1 activation but does not favor M2 differentiation. PLoS One. 2013;8:1–11.
Kang ML, Kim JE, Im GI. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65–78.
Ye J, Wang Q, Zhou X, Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Int J Pharm. 2008;352:273–9.
Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat Rev Rheumatol. 2017;13:183–93.
Bias P, Labrenz R, Rose P. Sustained-release dexamethasone palmitate: pharmacokinetics and efficacy in patients with activated inflammatory osteoarthritis of the knee. Clin Drug Investig. 2001;21:429–36.
Paik J, Duggan ST, Keam SJ. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79:455–62.
Goldberg VM, Goldberg L. Intra-articular hyaluronans: the treatment of knee pain in osteoarthritis. J Pain Res. 2010;3:51–6.
Strauss EJ, Hart J, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis. Am J Sports Med. 2009;37:1636–44.
Zhang Z, Wei X, Gao J, Zhao Y, Zhao Y, Guo L, et al. Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: an experimental study in a rat model of osteoarthritis. Int J Mol Sci. 2016;17.
Rutjes AWS, Ju P, Costa BR, Trelle S, Nu E. Annals of internal medicine viscosupplementation for osteoarthritis of the knee. Ann Intern Med. 2012;157:180–91.
Petit A, Redout EM, van de Lest CH, de Grauw JC, Müller B, Meyboom R, et al. Sustained intra-articular release of celecoxib from in situ forming gels made of acetyl-capped PCLA-PEG-PCLA triblock copolymers in horses. Biomaterials. 2015;53:426–36.
Turker S, Ozer AY, Kilic E, Ozalp M, Colak S, Korkmaz M. Gamma-irradiated liposome/noisome and lipogelosome/niogelosome formulations for the treatment of rheumatoid arthritis. Interv Med Appl Sci. 2013;5:60–9.
Lei Y, Rahim M, Ng Q, Segura T. Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery. J Control Release. 2011;153:255–61.
Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014.
Dion J, Deshayes F, Storozhylova N, Advedissian T, Lambert A, Viguier M, et al. Lactosamine-based derivatives as tools to delineate the biological functions of galectins: application to skin tissue repair. ChemBioChem. 2017;18:782–9.
Coppin L, Vincent A, Frénois F, Duchêne B, Lahdaoui F, Stechly L, et al. Galectin-3 is a non-classic RNA binding protein that stabilizes the mucin MUC4 mRNA in the cytoplasm of cancer cells. Sci Rep. 2017;7:43927.
Janelle-Montcalm A, Boileau C, Poirier F, Pelletier J-P, Guévremont M, Duval N, et al. Extracellular localization of galectin-3 has a deleterious role in joint tissues. Arthritis Res Ther. 2007;9:R20.
Hu Y, Yéléhé-Okouma M, Ea HK, Jouzeau JY, Reboul P. Galectin-3: a key player in arthritis. Jt Bone Spine. 2017;84:15–20.
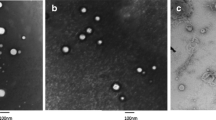
Crecente-Campo J, Alonso MJ. Engineering, on-demand manufacturing, and scaling-up of polymeric nanocapsules. Bioeng Transl Med. 2018;4:38–50.
Urban CMC, Mainardes RM, Gremião MPD. Development and validation of HPLC method for analysis of dexamethasone acetate in microemulsions. 2009;45.
Kupper CE, Rosencrantz RR, Henßen B, Pelantová H, Thönes S, Drozdová A, et al. Chemo-enzymatic modification of poly-N-acetyllactosamine (LacNAc) oligomers and N,N-diacetyllactosamine (LacDiNAc) based on galactose oxidase treatment. Beilstein J Org Chem. 2012;8:712–25.
Lutolf MP, Hubbell J a. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722.
Margareth RC, Raimar L. Simulated biologic fluids with possible application in dissolution testing. Dissolutio Technol. 2011:15–28.
Kaufman GN. Therapeutic potential of endothelin receptor type a and bradykinin receptor B1 dual antagonism in osteoarthritis treatment. Univ Montr. 2010.
Ekundi-Valentim E, Santos KT, Camargo E, Denadai-Souza, Teixeira S, Zanoni CI, et al. Differing effects of exogenous and endogenous hydrogen sulphide in carrageenan-induced knee joint synovitis in the rat. Br J Pharmacol. 2010;159:1463–74.
Morgen M, Tung D, Boras B, Miller W, Malfait AM, Tortorella M. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm Res. 2013;30:257–68.
Alonso MJ, Garcia-Fuentes M. Nano-Oncologicals: New Targeting and Delivery Approaches. 2014.
Clemons TD, Viola HM, House MJ, Hool LC, Iyer KS. The Design and Testing of Multifunctional Nanoparticles for Drug Delivery Applications. 2016.
Rothenfluh DA, Bermudez H, Neil CPO, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. 2008;7:1–7.
Prince A. Development of in situ forming hydrogels for intra-articular drug delivery. 2019.
Fezai M, Senovilla L, Jemaà M, Ben-Attia M, Ben-Attia M. Analgesic, anti-inflammatory and anticancer activities of extra virgin olive oil. J Lipids. 2013;2013:1–7.
Moghimipour E, Salimi A, Karami M, Isazadeh S. Preparation and characterization of dexamethasone microemulsion based on pseudoternary phase diagram. Jundishapur J Nat Pharm Prod. 2013;8:105–12.
Chime S, Kenechukwu FC, Attama A. Nanoemulsions — advances in formulation, characterization and applications in drug delivery. 2014.
Mourdikoudis S, Liz-Marzán LM. Oleyamine in nanoparticle synthesis. Chem Mater. 2013;25:1465.
Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37.
El-Hakim IES, Abdel-Hamid IS, Bader a. Tempromandibular joint (TMJ) response to intra-articular dexamethasone injection following mechanical arthropathy: a histological study in rats. Int J Oral Maxillofac Surg 2005;34:305–310.
Chen WS, Cao Z, Leffler H, Nilsson UJ, Panjwani N. Galectin-3 inhibition by a small-molecule inhibitor reduces both pathological corneal neovascularization and fibrosis. Investig Ophthalmol Vis Sci. 2017;58:9–20.
Chernyshev VS, Rachamadugu R, Tseng YH, Belnap DM, Jia Y, Branch KJ, et al. Size and shape characterization of hydrated and desiccated exosomes. Anal Bioanal Chem. 2015;407:3285–301.
Brown AC, Barker TH. Fibrin-based biomaterials: modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014;10:1502–14.
Snyder TN, Madhavan K, Intrator M, Dregalla RC, Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng. 2014;8:10.
Vilela C, Correia C, Oliveira JM, Sousa RA, Espregueira-Mendes J, Reis RL. Cartilage repair using hydrogels: a critical review of in vivo experimental designs. ACS Biomater Sci Eng. 2015;150813111234008.
Avner Yayon, Meital Ben-Dayan Bloch, Ezequiel Wexselblatt, Ron Arbel GA. A chondrogenic fibrin-ha hybrid proteoglycan for OA pain relief and cartilage preservation from bench to clinics. 2017.
Chemistry and Biology of Hyaluronan. H.G. Garg and C.A. Hales (Ed.) 2004. p. 624, Amsterdam: Elsevier Science.
Masuko K, Murata M, Yudoh K, Kato T, Nakamura H. Anti-inflammatory effects of hyaluronan in arthritis therapy: not just for viscosity. Int J Gen Med. 2009;2:77–81.
Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33.
Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Ther. 2003;5:54–67.
Lugovskoy EV. Molecular Mechanisms of formation and degradation of fibrin. Komissarenko S.V. (Ed.) 2003. p. 223. Kyiv: Naukova Dumka.
Chernysh IN, Nagaswami C, Weisel JW. Visualization and identification of the structures formed during early stages of fibrin polymerization. 2011;117:4609–4614.
Weigel PH, Frost SJ, LeBoeuf RD, McGary CT. The specific interaction between fibrin(ogen) and hyaluronan: possible consequences in haemostasis, inflammation and wound healing. 1989;143:248–61; discussion 261–4, 281–5.
LeBoeuf RD, Raja RH, Fuller GM, Weigel PH. Human fibrinogen specifically binds hyaluronic acid. J Biol Chem. 1986;261:12586–92.
Gobbo S, Petrella RJ. WO 2007/131546. Hyaluronic acid binary mixtures and therapeutic thereof 2007.
Mehta DP, Shodhan K, Modi RI, Ghosh PK. Sodium hyaluronate of defined molecular size for treating osteoarthritis. Curr Sci. 2007;92:209–13.
Castor BCW. Hyaluronic acid in human synovial effusions; a sensitive Indicator of altered connective tissue cell function during inflammation. Arthritis Rheum. 1966;9:783–94.
Francis W, Marder J. Increased resistance to plasmic degradation of fibrin with highly crosslinked alpha-polymer chains formed at high factor XIII concentrations. Blood. 1988;71.
Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931–72.
Tsurupa G, Yakovlev S, McKee P, Medved L. Noncovalent interaction of alpha(2)-antiplasmin with fibrin(ogen): localization of alpha(2)-antiplasmin-binding sites. Biochemistry. 2010;49:7643–51.
Palmer M, Stanford E, Murray MM. The effect of synovial fluid enzymes on the biodegradability of collagen and fibrin clots. Materials. 2011;4:1469–82.
Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen. 2014;22:579–93.
Finelli I, Chiessi E, Galesso D, Renier D, Paradossi G. A new viscosupplement based on partially hydrophobic hyaluronic acid: a comparative study. Biorheology. 2011;48:263–75.
Choi B, Loh XJ, Tan A, Loh CK, Ye E. Introduction to in situ forming hydrogels for biomedical applications. 2015.
Park SH, Cui JH, Park SR, Min BH. Potential of fortified fibrin/hyaluronic acid composite gel as a cell delivery vehicle for chondrocytes. Artif Organs. 2009;33:439–47.
Zhang Y, Heher P, Hilborn J, Redl H, Ossipov D. Hyaluronic acid-fibrin interpenetrating double network hydrogel prepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications. Acta Biomater. 2016;38:23–32.
Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface. 2009;6:1–10.
Frost SJ, Weigel PH. Binding of hyaluronic acid to mammalian fibrinogens. BBA - Gen Subj. 1990;1034:39–45.
Matsuzaki T, Matsushita T, Tabata Y, Saito T, Matsumoto T, Nagai K, et al. Intra-articular administration of gelatin hydrogels incorporating rapamycin-micelles reduces the development of experimental osteoarthritis in a murine model. Biomaterials. 2014;35:9904–11.
Türker S, Erdoǧan S, Özer AY, Ergün EL, Tuncel M, Bilgili H, et al. Scintigraphic imaging of radiolabelled drug delivery systems in rabbits with arthritis. Int J Pharm. 2005;296:34–43.
Webber MJ, Matson JB, Tamboli VK, Stupp SI. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33:6823–32.
Mosesson MW, Siebenlist KR, Hernandez I, Lee KN, Christiansen VJ, Mckee P a. Evidence that a2-antiplasmin becomes covalently ligated to plasma fibrinogen in the circulation: a new role for plasma factor XIII in fibrinolysis regulation. J Thromb Haemost. 2008;6:1565–1570.
Chen HY, Liu F-T, Yang R-Y. Roles of galectin-3 in immune responses. Arch Immunol Ther Exp. 2005;53:497–504.
Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue. Nat Rev Mol Cell Biol. 2007;8:221–33.
ClinicalTrials NCT02407041. An open-label, phase 2a study to evaluate safety and efficacy of GR-MD-02 for treatment of psoriasis. 2018.
Clinical Trials NCT02257177. RCT (randomized control trial) of TD139 vs placebo in HV’s (human volunteers) and IPF patients purpose. 2017.
Santos JM, Bárcia RN, Simões SI, Gaspar MM, Calado S, Agua-Doce A, et al. The role of human umbilical cord tissue-derived mesenchymal stromal cells (UCX®) in the treatment of inflammatory arthritis. J Transl Med. 2013;11:18.
Hsieh TJ, Lin HY, Tu Z, Huang BS, Wu SC, Lin CH. Structural basis underlying the binding preference of human galectins-1, -3 and -7 for Galβ1-3/4GlcNAc. PLoS One. 2015;10:1–19.
Hsieh T, Lin H-Y, Tu Z, Lin T-C, Wu S, Tseng Y, et al. Dual thio-digalactoside-binding modes of human galectins as the structural basis for the design of potent and selective inhibitors. Sci Rep. 2016;6:29457.
Giuseppe Cirino CC. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996;183.
Acknowledgements
The authors express gratitude for financial support from EM NanoFar Doctoral Fellowship (Project 2013-05-C2-EM), Xunta de Galicia (Competitive Reference Groups-FEDER Funds Ref: ED431C 2017/09, ENE2017-86425-C2-1-R Project), Xunta de Galicia and the Laboratoires Servier for post-doctoral fellowships, and Fundação para a Ciência e a Tecnologia (Program UID/DTP/04138/2013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supporting Information is available from the authors.
ESM 1
(DOCX 546 kb)
Rights and permissions
About this article
Cite this article
Storozhylova, N., Crecente-Campo, J., Cabaleiro, D. et al. An In Situ Hyaluronic Acid-Fibrin Hydrogel Containing Drug-Loaded Nanocapsules for Intra-Articular Treatment of Inflammatory Joint Diseases. Regen. Eng. Transl. Med. 6, 201–216 (2020). https://doi.org/10.1007/s40883-020-00154-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-020-00154-2