Abstract
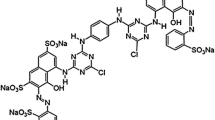
There are thousands of commercially available dyes which represent 7 × 105 tons produced annually worldwide. Discharges of these dyes from dyeing industry effluents have the potential to reach aquatic environments, affecting many life forms. Methylene Blue (MB) is the most common dye used in textile industries. Congo Red (CR) is an azo-based dye that can be metabolized in benzidine, a human carcinogen agent. In this study, polymeric beads made of chitosan and cellulose were used to remove simultaneously a cationic dye (MB) and a anionic dye (CR). The sorption capacity of the beads was evaluated using different dye concentrations under individual (MB or CR) and simultaneous (MB and CR) conditions. Dyes’ concentration in solution was determined by HPLC–DAD. The results evidenced that both dyes can be adsorbed individually and simultaneously on the polymeric beads. The sorption process fitted the Langmuir’s isotherm. The qmax values for CR and MB simultaneous adsorption were 1.6 and 0.68 µmol/g, respectively. The chitosan/cellulose beads exhibited a large adsorption capacity for CR on individual and simultaneous experiments. Adsorption mechanism involves electrostatic interactions, hydrogen bonds and dipole-dipole forces between the functional groups of the polymeric beads and the dyes.







Similar content being viewed by others
References
Chatterjee S, Lee DS, Lee MW, Woo SH (2009) Congo red adsorption from aqueous solutions by using chitosan hydrogel beads impregnated with nonionic or anionic surfactant. Bioresour Technol 100(17):3862–3868
Crespo-Alonso M, Nurchi VM, Biesuz R, Alberti G, Spano N, Pilo MI, Sanna G (2013) Biomass against emerging pollution in wastewater: ability of cork for the removal of ofloxacin from aqueous solutions at different pH. J Environ Chem Eng 1(4):1199–1204
Crini G, Badot P-M (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33(4):399–447
Eberhard FS, Hamawand I (2017) Selective electrodialysis for copper removal from brackish water and coal seam gas water. Int J Environ Res 11(1):1–11
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Gupta VK, Suhas VK (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90(8):2313–2342
Kosmulski M (2014) The pH dependent surface charging and points of zero charge. VI. Update. J Colloid Interface Sci 426:209–212
Lopez-Morales J, Perales-Perez O, Roman-Velazquez FR (2012) Sorption of triclosan onto tyre crumb rubber. Adsorpt Sci Technol 30(12):831–845
Luo X, Zhang L (2009) High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J Hazard Mater 171(1):340–347
Mahmoodi NM, Salehi R, Arami M, Bahrami H (2011) Dye removal from colored textile wastewater using chitosan in binary systems. Desalination 267(1):64–72
Moosavian MA, Moazezi N (2016) Removal of cadmium and zinc ions from industrial wastewater using nanocomposites of PANI/ZnO and PANI/CoHCF: a comparative study. Desalin Water Treat 57(44):20817–20836
Musyoka SM, Mittal H, Mishra SB, Ngila JC (2014) Effect of functionalization on the adsorption capacity of cellulose for the removal of methyl violet. Int J Biol Macromol 65:389–397
Nurchi VM, Crespo-Alonso M, Biesuz R, Alberti G, Pilo MI, Spano N, Sanna G (2014) Sorption of chrysoidine by row cork and cork entrapped in calcium alginate beads. Arab J Chem 7(1):133–138
Osifo PO, Webster A, van der Merwe H, Neomagus HW, van der Gun MA, Grant DM (2008) The influence of the degree of cross-linking on the adsorption properties of chitosan beads. Bioresour Technol 99(15):7377–7382
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1):70–80
Salleh MAM, Mahmoud DK, Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1):1–13
Wan Ngah WS, Teong LC, Hanafiah M (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83(4):1446–1456
Yan H, Zhang W, Kan X, Dong L, Jiang Z, Li H, Yang H, Cheng R (2011) Sorption of methylene blue by carboxymethyl cellulose and reuse process in a secondary sorption. Colloids Surf A Physicochem Eng Asp 380(1):143–151
Acknowledgements
The authors are thankful for the financial support from the US Department of Agriculture through the Center for Education and Training in Agricultural and Related Sciences Proposal (CETARS; award #: USDA/NIFA: 2011-38422-30835), at Inter American University of Puerto Rico at San German and University of Puerto Rico at Mayaguez. Special thanks to Mrs. Nilda Caraballo (Inter American University of Puerto Rico) and Mrs. Marta Caraballo (Fenwal: A Fresenius Kabi Company).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vega-Negron, A.L., Alamo-Nole, L., Perales-Perez, O. et al. Simultaneous Adsorption of Cationic and Anionic Dyes by Chitosan/Cellulose Beads for Wastewaters Treatment. Int J Environ Res 12, 59–65 (2018). https://doi.org/10.1007/s41742-018-0066-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-018-0066-2