Abstract
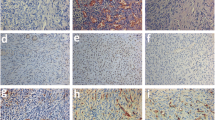
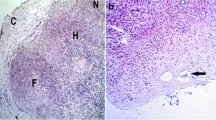
This study evaluated the effect of adding alpha lipoic acid (ALA) to the vitrification solution of sheep ovarian tissue on 7 days of in vitro culture or 15 days of xenotransplantion. ALA was used at two different concentrations (100 μM: ALA100 and 150 μM: ALA150). Ovarian tissue was evaluated by classical histology (follicular morphology, development, and stromal cell density); immunohistochemistry for forkhead box O3a (FOXO3a); Ki67 (cell proliferation); cluster of differentiation 31 (CD31); and alpha smooth muscle actin (α-SMA). Reactive oxygen species (ROS) levels in ovarian tissue, as well as malondialdehyde (MDA) and nitrite levels in the culture medium, were assessed. Similar percentage of morphologically normal follicles was found in the vitrified ovarian tissue in the presence of ALA100 or ALA150 after in vitro culture or xenotransplantation. Follicular development from all treatments was higher (P < 0.05) than the control group. Moreover, an activation of primordial follicles was observed by FOXO3a. Stromal cell density and immunostaining for Ki67 and CD31 were significantly higher (P < 0.05) in ALA150 vitrified tissue. No difference (P > 0.05) was found in α-SMA between ALA concentrations after in vitro culture or xenograft. ROS levels in the ovarian tissue were similar (P > 0.05) in all treatments, as well as MDA and nitrite levels after 7 days of culture. We concluded that the addition of ALA 150 is able to better preserve the stromal cell density favoring granulosa cell proliferation and neovascularization.







Similar content being viewed by others
Data Availability
Not applicable.
References
Mottet A, Teillard F, Boettcher P, De’ Besi G, Besbes B. Domestic herbivores and food security: current contribution, trends and challenges for a sustainable development. Animal. 2018;12(2):188–98.
Lôbo RNB, Pereira IDC, Facó O, Mc Manus CM. Economic values for production traits of Morada Nova meat sheep in a pasture based production system in semi-arid Brazil. Small Rum Res. 2011;96:93–100.
Facó O, Paiva SR, Alves LRN, Lobo RNB, Villela LCV. Raça Morada Nova: origem, características e perspectivas. Embrapa. 2008. http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/533728. Accesed 15 Jan 2020.
Figueiredo JR., Rodrigues APR., Amorim CA, Silva JRV. Manipulação de oócitos inclusos em folículos ovarianos pré-antrais - MOIFOPA. In: Gonçalves PBD, Figueiredo JR, Freitas VJF, editors. Biotécnicas aplicadas à reprodução animal. São Paulo: Roca; 2008. pp.303–27.
Shaw JM, Oranratnachai A, Trounson, AO. Fundamental cryobiology of mammalian oocytes and ovarian tissue. Theriogenology. 2000; 53(1):59–72.
Anderson RA, Wallace WHB, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reproduction. 2008;136:681–9.
Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603.
Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen-thawed hemi-ovaries into ewes. Fertil Steril. 2002;77:403–8.
Salle B, Demirci B, Franck M, Berthollet C, Lornage J. Long-term follow-up of cryopreserved hemi-ovary autografts in ewes: pregnancies, births, and histologic assessment. Fertil Steril. 2003;80:172–7.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–10.
Dolmans MM, Falcone T, Patrizio P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil Steril. 2020;114:279–80.
Bordes A, Lornage J, Demirci B, Franck M, Courbiere B, Guerin JF, et al. Normal gestations and live births after orthotopic autograft of vitrified-warmed hemi-ovaries into ewes. Hum Reprod. 2005;20:2745–8.
Kawamura N, Tamuraa M, Hashimotoe S, Sugishitaa Y, Morimotoe Y, Hosoif Yoshioka N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. The Jackson Laboratory. 2013;110(43):17474–9.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608–15.
Kazemein Jasemi VS, Samadi F, Eimani H, Hasani S, Fathi R, Shahverdi A, et al. Function of vitrified mouse ovaries tissue under static magnetic field after autotransplantation. Vet Res Forum. 2017;8(3):243–9.
Fujihara M, Kaneko T, Inoue M. Vitrification of canine ovarian tissues with polyvinylpyrrolidone preserves the survival and developmental capacity of primordial follicles. Scientific Reports. 2019;9:3970.
Marques LS, Fossati AAN, Rodrigues RB, Da Rosa HT, Izaguirry AP, Ramalho JB, et al. Slow freezing versus vitrification for the cryopreservation of zebrafish (Danio rerio) ovarian tissue. Scientific Reports. 2019;9:15353.
Melo MA, Oskam IC, Celestino JJ, Carvalho AA, Castro SV, Figueiredo JR, et al. Adding ascorbic acid to vitrification and IVC medium influences preantral follicle morphology, but not viability. Reprod Domest Anim. 2011;46(4):742–5.
Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, et al. Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system. Hum Reprod. 2013;28(5):1267–79.
Carvalho AA, Faustino LR, Silva CMG, Castro SV, Lobo CH, Santos FW, Santos RR, Campello CC, Bordignon V, Figueiredo JR, Rodrigues APR. Catalase addition to vitrification solutions maintains goat ovarian preantral follicles stability. Res Vet Sci. 2014;97:140–7.
Dos Santos Morais MLG, de Brito DCC, Pinto Y, Mascena Silva L, Montano Vizcarra D, Silva RF, et al. Natural antioxidants in the vitrification solution improve the ovine ovarian tissue preservation. Reprod Biol. 2019;19(3):270–8.
Schmidt AM, Hori O, Brett J, Yan SD, Wautier JL, Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14(10):1521–8.
Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19(2):227–50.
Gomes RG, Silva CB, Gonzalez SM, Oliveira RL, Max MC, Lisboa LA, et al. Alpha lipoic acid (ALA) effects on developmental competence of equine preantral follicles in short-term culture. Theriogenology. 2018;105:169–73.
Hatami S, Zavareh S, Salehnia M, Lashkarbolouki T, Ghorbanian MT, Karimi I. Total oxidative status of mouse vitrified pre-antral follicles with pre-treatment of alpha lipoic acid. Iran Biomed J. 2014;18(3):181–8.
Zoheir KM, Harisa GI, Allam AA, Yang L, Li X, Liang A, et al. Effect of alpha lipoic acid on in vitro development of bovine secondary preantral follicles. Theriogenology. 2017;88:124–30.
Silva LM, Mbemya GT, Guerreiro DD, Brito DCC, Donfack NJ, Morais MLGS, et al. Effect of catalase or alpha lipoic acid supplementation in the vitrification solution of ovine ovarian tissue. Biopreserv Biobank. 2018;16:258–69.
Carvalho AA, Faustino LR, Silva CM, Castro SV, Lopes CA, Santos RR, et al. Novel wide-capacity method for vitrification of caprine ovaries: Ovarian Tissue Cryosystem (OTC). Anim Reprod Sci. 2013;138(3-4):220–7.
Lunardi FO, Chaves RN, de Lima LF, Araújo VR, Brito IR, Souza CEA, et al. Vitrified sheep isolated secondary follicles are able to grow and form antrum after a short period of in vitro culture. Cell Tissue Res. 2015;362:241–51.
Wang L, Ying Y, Ouyang Y, Wang J, Xu J. VEGF and bFGF increase survival of xenografted human ovarian tissue in an experimental rabbit model. J Assist Reprod Genet. 2013;30:1301–11.
Nichols-Burns SM, Lotz L, Schneider H, Adamek E, Daniel C, Stief A, Grigo C, Klump D, et al. Preliminary observations on whole-ovary xenotransplantation as an experimental model for fertility preservation. Reprod Biomed Online. 2014;29(5):621–6.
Silva JRV, Ferreira MAL, Costa SHF, Santos RR, Carvalho FCA, Rodrigues APR, et al. Degeneration rate of preantral follicles in the ovaries of goats. Small Rum Res. 2002;43:203–9.
Silva JRV, Van Den Hurk R, De Matos MHT, Dos Santos RR, Pessoa C, De Moraes MO, et al. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology. 2004;61:1691–704.
Alves KA, Alves BG, Gastal GDA, Tarso SGS, Gastal MO, Figueiredo JR, et al. The Mare Model to Study the Effects of Ovarian Dynamics on Preantral Follicle Features. PLoS One. 2016;11(2):1–18.
Ting AY and Zelinski MB. Characterization of FOXO1, 3 and 4 transcription factors in ovaries of fetal, prepubertal and adult rhesus macaques. Biol Reprod. 2017;96(5):1052–9.
Hewitt SM, Baskin DG, Frevert CW, Stahl WL, Rosa-Molinar E. Controls for Immunohistochemistry: The Histochemical Society’s Standards of Practice for Validation of Immunohistochemical Assays. J Histochem Cytochem. 2014;62(10):693–7.
Uhlenhaut NH, Treier M. Forkhead transcription factors in ovarian function. Reproduction. 2011;142(4):489–95.
Scalercio SR, Amorim CA, Brito DC, Percário S, Oskam IC, Domingues SFS, et al. Trolox enhances follicular survival after ovarian tissue autograft in squirrel monkey (Saimiri collinsi). Reprod Fertil and Dev. 2015;28(11):1854–64.
Loetchutinat C, Kothan S, Dechsupa S, Meesungnoen J, Jay-Gerin JP, Mankhetkorn S. Spectrofluorometric determination of intracellular levels of reactive oxygen species in drug-sensitive and drug-resistant cancer cells using the 2′,7′-dichlorofluorescein diacetate assay. Radiat Phys Chem. 2005;72:323–31.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and nitrate in biological fluids. Anal Biochem. 1982;126(1):131–8.
Draper HH, Hadely M. Malondialdehyde determination as an índex of lipid peroxidation. Meth. Enzymol. 1990;186:421–43.
Rahimi G, Isachenko E, Sauer H, Isachenko V, Wartenberg M, Hescheler J, et al. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod Fertil Dev. 2003;15(6):343–9.
Amoushahi M, Salehnia M. Reactive oxygen species level, mitochondrial transcription factor A gene expression and succinate dehydrogenase activity in metaphase II oocytes derived from in vitro cultured vitrified mouse ovaries. Vet Res Forum. 2018;9(2):145–52.
Montano Vizcarra DA, Pinto Silva Y, Bezerra Bruno J, Calado Brito DC, Dipaz Berrocal D, Mascena Silva L, et al. Use of synthetic polymers improves the quality of vitrified caprine preantral follicles in the ovarian tissue. Acta Histochem. 2020;122(2):151484.
Bandeira F, Carvalho A, Castro S, Lima L, Viana D, Evangelista J, et al. Two Methods of Vitrification Followed by In Vitro Culture of the Ovine Ovary: Evaluation of the Follicular Development and Ovarian Extracellular Matrix. Reprod Domest Anim. 2014;50(2):177–85.
Tahaei LS, Eimani H, Hajmusa G, Fathi R, Rezazadeh Valojerdi M, Shahverdi A, Eftekhari-Yazdi P. Follicle Development of Xenotransplanted Sheep Ovarian Tissue into Male and Female Immunodeficient Rats. Int J Fertil Steril. 2015;9(3):354–60.
Jiatsa Donfack N, Alves KA, Alves BG, Pedrosa Rocha RM, Bruno JB, Lobo CH, et al. Xenotransplantation of goat ovary as an alternative to analyse follicles after vitrification. Reprod Domest Anim. 2019;54(2):216–24.
Aye M, Giorgio CD, Mo MD, Botta A, Perrin J, Courbiere B. Assessment of the genotoxicity of three cryoprotectants used for human oocyte vitrification: Dimethyl sulfoxide, ethylene glycol and propylene glycol. Food Chem Toxicol. 2010;48:1905–12.
Oskam IC, Lund T, Santos RR. Irreversible damage in ovine ovarian tissue after cryopreservation in propanediol: analyses after in vitro culture and xenotransplantation. Reprod Domest Anim. 2011;46(5):793–9.
Lee SW, Otsuka F, Moore KR and Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the Rat. Biol Reprod. 2001;65(4):994–9.
Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2001;141(6):809–20.
Rezk BM, Haenen GR, van der Vijgh WJ, Bast A. Lipoic acid protects efficiently only against a specific form of peroxynitrite-induced damage. J. Biol Chem. 2004;279:9693–7.
Maeda A, Crabb JW, Palczewski K. Microsomal glutathione S-transferase 1 in the retinal pigment epithelium: protection against oxidative stress and a potential role in aging. Biochemistry. 2005;44:480–9.
Yang Y, Yang Y, Trent MB, He N, Lick SD, Zimniak P, et al. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: possible role in human atherosclerosis: Possible role in human atherosclerosis. Atherosclerosis. 2004;173:211–21.
Ibrahim SF, Osman K, Das S, Othman AM, Majid NA, Rahman MPA. A study of the antioxidant effect of alpha lipoic acids on sperm quality. Clinics (São Paulo). 2008;63(4):545–50.
Hardy K, Mora JM, Dunlop C, Carzaniga C, Franks S, Fenwick MA. Nuclear exclusion of smad2/3 in granulosa cells is associated with primordial follicle activation in the mouse ovary. J Cell Sci. 2018;131(17).
Ramezani M, Salehnia M, Jafarabadi M. Short Term Culture of Vitrified Human Ovarian Cortical Tissue to Assess the Cryopreservation Outcome: Molecular and Morphological Analysis. J Reprod Infertil. 2017;18(1):162-171.
Dolmans MM, Cordier F, Amorim CA, Donnez J, Linden CV. In vitro activation prior to transplantation of human ovarian tissue: Is it truly effective? Front Endocrinol (Lausanne). 2019;10:520.
Bertoldo MJ, Walters KA, Ledger WL, Gilchrist RB, Mermillod P, Locatelli Y. In-vitro regulation of primordial follicle activation: challenges for fertility preservation strategies. Reprod Biomed Online. 2018;36:491–9.
Kim JY. Control of ovarian primordial follicle activation. Clin Exp Reprod Med 2012;39(1):10–4.
Figueiredo JR, Lima LF, Silva JR, Santos RR. Control of growth and development of preantral follicle: insights from in vitro culture. Anim Reprod. 2018;15(1):648–59.
Scalercio SRRA, Brito AB, Domingues SFS, Santos RR, Amorim CA. Immunolocalisation of growth, inhibitory and proliferative factors involved in initial ovarian folliculogenesis from adult common squirrel monkey (Saimiri sciureus). Reprod. Sci. 2015;22(1):68–74.
Du L, Miao X, Gao Y, Jia H, Liu K, Liu Y. The protective effects of Trolox-loaded chitosan nanoparticles against hypoxiamediated cell apoptosis. Nanomedicine. 2014;10(7):1411–20.
Cakatay U. Pro-oxidant actions of alpha-lipoic acid and dihydrolipoic acid. Med Hypotheses. 2006;66:110.
Donfack JN, Alves AK, Alves GB, Rocha PMR, Bruno BJ, et al. In vivo and in vitro strategies to support caprine preantral follicle development after ovarian tissue vitrification. Reprod Fertil Dev. 2018;30(8):1055–65.
Choi WJ, Lee JH, Park MJ, Choi IY, Park JK, Shin JK, et al. Influence of the vitrification solution on the angiogenic factors in vitrificated mouse ovarian tissue. Obstec Gynecol Sci. 2013;56(6):382–8.
Henry L, Labied S, Fransolet M, Kirschvink N, Blacher S, Noel A, et al. Isoform 165 of vascular endothelial growth factor in collagen matrix improves ovine cryopreserved ovarian tissue revascularization after xenotransplantation in mice. Reprod Biol Endocrinol. 2015;13:12.
Van Eyck AS, Bouzin C, Feron O, Romeu L, Van Langendonckt A, Donnez J, et al. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil Steril. 2010;93:1676–85.
Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M. Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrast-enhanced MRI. Magn Reson Med. 2004;52:741–50.
Steif PS, Matthew C. Palastro, and Yoed Rabin. Analysis of the Effect of Partial Vitrification on Stress Development in Cryopreserved Blood Vessels. Med Eng Phys. 2007;29(6):661–70.
Li F, Sawada J, Komatsu M. R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Nat commun. 2017;8(1):1720.
Castrillon DH, Miao L, Kollipara R, Horner JW, De Pinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301(5630):215–8.
Visser J A, de Jong FH, Laven JS, Themmen AP. Anti- Mu¨llerian hormone: a new marker for ovarian function. Reproduction. 2006;131(1):1–9.
Giaretta E, Spinaci M, Bucci D, Tamanini C, Galeati G. Effects of resveratrol on vitrified porcine oocytes. Oxidat Med and Cell Longev. 2013;2013:920257.
Talebi A, Zavareh S, Kashani MH, Lashgarbluki T, Karimi I. The effect of alpha lipoic acid on the developmental competence of mouse isolated preantral follicles. J Assist Reprod Genet. 2012;29(2):175–83.
Zavareh S, Karimi I, Salehnia M, Rahnama A. Effect of In Vitro Maturation Technique and Alpha Lipoic Acid Supplementation on Oocyte Maturation Rate: Focus on Oxidative Status of Oocytes. Int J Fertil Steril. 2016;9(4):442–51.
Moini H, Packer L, Saris NE. Antioxidant and prooxidant activities of alpha lipoic acid and dihydrolipoic acid. Toxicol Appl Pharmacol. 2002;182:84–90.
Armagan I, Bayram D, Candan IA, et al. Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environ Toxicol. Pharmacol. 2015;39:1122–31.
Bilska A, Wlodek L. Lipoic acid - the drug of the future? Pharmacol Rep. 2005;57(5):570–7.
Acknowledgements
We would like to thank Eastman G. Welsford DMV for proofreading the manuscript.
Funding
This work was financed by The National Council of Technological and Scientific Development (CNPq: 433.262/2016-8). Lucy Vanessa Sulca Ñaupas is a recipient of a grant from CAPES. Ana Paula Ribeiro Rodrigues is the recipient of a grant (number of the process: 308.071/2016-6) from CNPq.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics Approval
This study was approved and performed under the guidelines of the Ethics Committee for Animal Use of the State University of Ceará (4156483/2018) and University of Fortaleza (9554130618/2018).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ñaupas, L.V.S., Brito, D.C.C., de Souza, S.S. et al. Alpha Lipoic Acid Supplementation Improves Ovarian Tissue Vitrification Outcome: An Alternative to Preserve the Ovarian Function of Morada Nova Ewe. Reprod. Sci. 28, 3109–3122 (2021). https://doi.org/10.1007/s43032-021-00593-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00593-4