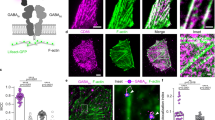
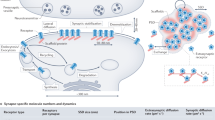
Abstract
Variations in receptor number at a given synapse are known to contribute to synaptic plasticity, but methods used to establish this idea usually do not allow for the determination of the dynamics of these phenomena. We used single-particle tracking to follow in real time, on the cell surface, movements of the glycine receptor (GlyR) with or without the GlyR stabilizing protein gephyrin. GlyR alternated within seconds between diffusive and confined states. In the absence of gephyrin, GlyR were mostly freely diffusing. Gephyrin induced long confinement periods spatially associated with submembranous clusters of gephyrin. However, even when most receptors were stabilized, they still frequently made transitions through the diffusive state. These data show that receptor number in a cluster results from a dynamic equilibrium between the pools of stabilized and freely mobile receptors. Modification of this equilibrium could be involved in regulation of the number of receptors at synapses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Felsenfeld, D. P., Choquet, D. P. & Sheetz, M. P. Ligand binding regulates the directed movement of β1 integrins on fibroblasts. Nature 383, 438–440 (1996).
Qian, H., Sheetz, M. P. & Elson, E. L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 60, 910–921 (1991).
Sako, Y. & Kusumi, A. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J. Cell Biol. 125, 1251–1264 (1994).
Kusumi, A., Sako, Y. & Yamamoto, M. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy). Effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J. 65, 2021–2040 (1993).
Saxton, M. J. & Jacobson, K. Single-particle tracking: applications to membrane dynamics. Annu. Rev. Biophys. Biomol. Struct. 26, 373–399 (1997).
Kuromi, H., Brass, B. & Kidokoro, Y. Formation of acetylcholine receptor clusters at neuromuscular junction in Xenopus cultures. Dev. Biol. 109, 165–176 (1985).
Froehner, S. C. Regulation of ion channel distribution at synapses. Annu. Rev. Neurosci. 16, 347–368 (1993).
Craig, A. M., Blackstone, C. D., Huganir, R. L. & Banker, G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc. Natl. Acad. Sci. USA 91, 12373–12377 (1994).
Turrigiano, G. G. AMPA receptors unbound: membrane cycling and synaptic plasticity. Neuron 26, 5–8 (2000).
Scannevin, R. H. & Huganir, R. L. Postsynaptic organization and regulation of excitatory synapses. Nat. Rev. Neurosci. 1, 133–141 (2000).
Ziff, E. B. Enlightening the postsynaptic density. Neuron 19, 1163–1174 (1997).
Luthi, A. et al. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF–GluR2 interaction. Neuron 24, 389–399 (1999).
Carroll, R. C., Lissin, D. V., von Zastrow, M., Nicoll, R. A. & Malenka, R. C. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat. Neurosci. 2, 454–460 (1999).
Hayashi, Y. et al. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 (2000).
Nusser, Z., Hajos, N., Somogyi, P. & Mody, I. Increased number of synaptic GABA(A) receptors underlies potentiation at hippocampal inhibitory synapses. Nature 395, 172–177 (1998).
Sanes, J. R. & Lichtman, J. W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 22, 389–442 (1999).
Akaaboune, M., Culican, S. M., Turney, S. G. & Lichtman, J. W. Rapid and reversible effects of activity on acetylcholine receptor density at the neuromuscular junction in vivo. Science 286, 503–507 (1999).
Vannier, C. & Triller, A. Biology of the postsynaptic glycine receptor. Int. Rev. Cytol. 176, 201–244 (1997).
Betz, H. Structure and function of inhibitory glycine receptors. Q. Rev. Biophys. 25, 381–394 (1992).
Triller, A., Cluzeaud, F., Pfeiffer, F., Betz, H. & Korn, H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J. Cell Biol. 101, 683–688 (1985).
Todd, A. J. An electron microscope study of glycine-like immunoreactivity in laminae I-III of the spinal dorsal horn of the rat. Neuroscience 39, 387–394 (1990).
Kirsch, J., Wolters, I., Triller, A. & Betz, H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature 366, 745–748 (1993).
Meyer, G., Kirsch, J., Betz, H. & Langosch, D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron 15, 563–572 (1995).
Kirsch, J. & Betz, H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J. Neurosci. 15, 4148–4156 (1995).
Levi, S., Vannier, C. & Triller, A. Strychnine-sensitive stabilization of postsynaptic glycine receptor clusters. J. Cell Sci. 111, 335–345 (1998).
Kirsch, J. & Betz, H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature 392, 717–720 (1998).
Meier, J., Meunier-Durmort, C., Forest, C., Triller, A. & Vannier, C. Formation of glycine receptor clusters and their accumulation at synapses. J. Cell Sci. 113, 2783–2795 (2000).
Saxton, M. J. Lateral diffusion in an archipelago. Single-particle diffusion. Biophys. J. 64, 1766–1780 (1993).
Simson, R., Sheets, E. D. & Jacobson, K. Detection of temporary lateral confinement of membrane proteins using single-particle tracking analysis. Biophys. J. 69, 989–893 (1995).
Saffman, P. G. Brownian motion in thin sheets of viscous fluid. J. Fluid Mech. 73, 593–602 (1976).
Kucik, D. F., Elson, E. L. & Sheetz, M. P. Weak dependence of mobility of membrane protein aggregates on aggregate size supports a viscous model of retardation of diffusion. Biophys. J. 76, 314–322 (1999).
Bechade, C., Colin, I., Kirsch, J., Betz, H. & Triller, A. Expression of glycine receptor alpha subunits and gephyrin in cultured spinal neurons. Eur. J. Neurosci. 8, 429–435 (1996).
Colin, I., Rostaing, P., Augustin, A. & Triller, A. Localization of components of glycinergic synapses during rat spinal cord development. J. Comp. Neurol. 398, 359–372 (1998).
Colin, I., Rostaing, P. & Triller, A. Gephyrin accumulates at specific plasmalemma loci during neuronal maturation in vitro. J. Comp. Neurol. 374, 467–479 (1996).
Levi, S., Chesnoy-Marchais, D., Sieghart, W. & Triller, A. Synaptic control of glycine and GABA(A) receptors and gephyrin expression in cultured motoneurons. J. Neurosci. 19, 7434–7449 (1999).
Dumoulin, A., Levi, S., Riveau, B., Gasnier, B. & Triller, A. Formation of mixed glycine and GABAergic synapses in cultured spinal cord neurons. Eur. J. Neurosci. 12, 3883–3892 (2000).
Kirsch, J. Assembly of signaling machinery at the postsynaptic membrane. Curr. Opin. Neurobiol. 9, 329–335 (1999).
Kneussel, M. et al. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J. Neurosci. 19, 9289–9297 (1999).
Oda, Y., Charpier, S., Murayama, Y., Suma, C. & Korn, H. Long-term potentiation of glycinergic inhibitory synaptic transmission. J. Neurophysiol. 74, 1056–1074 (1995).
Charpier, S., Behrends, J. C., Triller, A., Faber, D. S. & Korn, H. “Latent” inhibitory connections become functional during activity-dependent plasticity. Proc. Natl. Acad. Sci. USA 92, 117–120 (1995).
Morishita, W. & Sastry, B. R. Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. J. Neurophysiol. 76, 59–68 (1996).
Essrich, C., Lorez, M., Benson, J. A., Fritschy, J. M. & Lüscher, B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1, 563–571 (1998).
Grenningloh, G. et al. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron 4, 963–970 (1990).
Grenningloh, G. et al. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature 328, 215–220 (1987).
Sontheimer, H. et al. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron 2, 1491–1497 (1989).
Meunier-Durmort, C., Grimal, H., Sachs, L. M., Demeneix, B. A. & Forest, C. Adenovirus enhancement of polyethylenimine-mediated transfer of regulated genes in differentiated cells. Gene Ther. 4, 808–814 (1997).
Choquet, D., Felsenfeld, D. P. & Sheetz, M. P. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell 88, 39–48 (1997).
Acknowledgements
We thank P. Ascher, A. Prochiantz and R. Miles for critical reading of the manuscript. Supported by grants from the CNRS, the INSERM, the Fondation pour la Recherche Médicale, the Association Française contre les Myopathies and the council of the Région Aquitaine. J.M. was supported by fellowships from Fonds der Chemischen Industrie (FCI; No. 0653082) and Deutscher Akademischer Austauschdienst (DAAD; No. D/98/03816) and Centre international des étudiants et stagiaires (CIES; No.242708G).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meier, J., Vannier, C., Sergé, A. et al. Fast and reversible trapping of surface glycine receptors by gephyrin. Nat Neurosci 4, 253–260 (2001). https://doi.org/10.1038/85099
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/85099
This article is cited by
-
Quantifying postsynaptic receptor dynamics: insights into synaptic function
Nature Reviews Neuroscience (2023)
-
Gephyrin-mediated formation of inhibitory postsynaptic density sheet via phase separation
Cell Research (2021)
-
Super-resolution microscopy: a closer look at synaptic dysfunction in Alzheimer disease
Nature Reviews Neuroscience (2021)
-
Electrodiffusion phenomena in neuroscience: a neglected companion
Nature Reviews Neuroscience (2017)
-
GABAA receptor dependent synaptic inhibition rapidly tunes KCC2 activity via the Cl−-sensitive WNK1 kinase
Nature Communications (2017)