Abstract
Correlation between blood pressure (BP) and target organ damage, vascular risk and long-term patient prognosis is greater for measurements derived from around-the-clock ambulatory BP monitoring than in-clinic daytime ones. Numerous studies consistently substantiate the asleep BP mean is both an independent and a much better predictor of cardiovascular disease (CVD) risk than either the awake or 24 h means. Sleep-time hypertension is much more prevalent than suspected, not only in patients with sleep disorders, but also among those who are elderly or have type 2 diabetes, chronic kidney disease or resistant hypertension. Hence, cost-effective adequate control of sleep-time BP is of marked clinical relevance. Ingestion time, according to circadian rhythms, of hypertension medications of six different classes and their combinations significantly affects BP control, particularly sleep-time BP, and adverse effects. For example, because the high-amplitude circadian rhythm of the renin–angiotensin–aldosterone system activates during nighttime sleep, bedtime vs. morning ingestion of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers better reduces the asleep BP mean, with additional benefit, independent of medication terminal half-life, of converting the 24 h BP profile into more normal dipper patterning. The MAPEC (Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares) study, first prospective randomized treatment-time investigation designed to test the worthiness of bedtime chronotherapy with ⩾1 conventional hypertension medications so as to specifically target attenuation of asleep BP, demonstrated, relative to conventional morning therapy, 61% reduction of total CVD events and 67% decrease of major CVD events, that is, CVD death, myocardial infarction, and ischemic and hemorrhagic stroke. The MAPEC study, along with other earlier conducted less refined trials, documents the asleep BP mean is the most significant prognostic marker of CVD morbidity and mortality; moreover, it substantiates attenuation of the asleep BP mean by a bedtime hypertension treatment strategy entailing the entire daily dose of ⩾1 hypertension medications significantly reduces CVD risk in both general and more vulnerable hypertensive patients, that is, those diagnosed with chronic kidney disease, diabetes and resistant hypertension.
Similar content being viewed by others
Introduction
The biology of human beings is not constant as inferred by the concept of homeostasis but variable in a predictable manner over time and manifested as rather precise and integrated oscillations of 24 h, week, month and year.1 These bioperiodicities and their underlying time-keeping mechanisms constitute a genetically fixed adaptive strategy enabling life forms to anticipate cyclic demands and challenges imposed by the environment, thereby conferring survival advantage in terms of energy economy, health preservation and species propagation. Circadian (~24 h) rhythms, which are particularly relevant to clinical medicine, are generated and regulated by an inherited central brain clock, the paired suprachiasmatic nuclei of the hypothalamus, that communicates via endocrine and neural signals to orchestrate and coordinate the multitude of other inherited clock networks at the molecular to organ-system level, thereby giving rise to a highly organized circadian time structure.2, 3 Blood pressure (BP) exhibits a mostly predictable 24 h pattern characterized in many, but not all, individuals with normotension or uncomplicated essential hypertension by: (1) striking morning-time BP rise, (2) two daytime peaks—the first ~2–3 h after awakening and the second late afternoon/early evening, (3) small mid-afternoon nadir and (4) 10–20% decline during sleep relative to the wake-time mean, the magnitude of decline being greater for systolic BP (SBP) than diastolic BP (DBP).4, 5, 6, 7 This awake/asleep pattern results from the interrelationship of many 24 h cyclic physiologic, neuroendocrine and environmental determinants: (1) rest activity-associated changes in behavior (including activity routine and level, fluid and stimulant (for example, caffeine) consumption, meal timings and content, emotional and mental stress and posture); (2) external day–night divergence in ambient light intensity and spectrum, temperature, humidity and noise; and (3) endogenous circadian (~24 h) variation in neuroendocrine, endothelial, vasoactive peptide and opioid and hemodynamic parameters (for example, plasma noradrenaline and adrenaline (autonomic nervous system), atrial natriuretic and calcitonin gene-related peptides and renin, angiotensin and aldosterone (renin–angiotensin–aldosterone system (RAAS)).7, 8, 9, 10 The usually higher awake BP to large extent thus derives from the high-amplitude circadian variation of sympathetic tone that peaks during the daytime as evidenced by highest concentrations of plasma noradrenaline and adrenaline after morning awakening and initial hours of the diurnal activity span,11 and also elements of the RAAS—prorenin, plasma renin activity, angiotensin-converting enzyme (ACE), angiotensin I and II, and aldosterone12—that all peak during the middle and latter span of nighttime sleep, independent of the daytime/nighttime alternation of posture.13 The normal lower BP during nighttime sleep relative to daytime wakefulness thus represents the combined simultaneous influence of predicable-in-time behavioral and environmental cycles plus circadian stage-dependent alterations, most prominently decline of sympathetic and rise of vagal tone, elevation of atrial natriuretic and calcitonin gene-related vasoactive peptides and depression of the RAAS during the first half of the rest span followed by progressive activation during the second half of rest until peaking in the morning.7, 9, 12, 14, 15, 16, 17
The fields of medical chronobiology (biological rhythms and medicine), chronopharmacology (impact on pharmacokinetics (PK) and pharmacodynamics (PD) according to drug administration time relative to biological rhythm stage) and chronotherapeutics (timing of conventional or special drug-delivery medication systems relative to biological rhythms to optimize beneficial and/or minimize/avert adverse effects) are growing in popularity. In order to make sound and useful scientific contributions to the fields of chronopharmacology and chronotherapeutics, it is necessary to decide which specific feature(s) of the 24 h BP profile is (are) worthy of targeting, not only to improve SBP and DBP control, but most importantly avert over time progressive injury to tissues and organs at highest risk, such as the blood vessels, brain, heart, kidney and retina, and also risk for nonfatal and fatal cerebrovascular (stroke) and cardiovascular disease (CVD) events. In addition, the choice of the most appropriate ingestion time should be based not only on achieving high patient compliance but also on how drug ingestion time with reference to the staging of key rhythms of the circadian time structure affects drug PK and PD. Given the differences between human beings in their bed and wake times and resulting corresponding disparities in staging of circadian rhythms that can influence the response to medication, we wish to emphasize that clock time is not equivalent to biological time, nor is it an accurate indicator of it. This is of the utmost fundamental relevance not only to circadian rhythm research and the clinical trialing of medications but also interpretation of study findings.18
This review presents the latest findings pertaining to bedtime hypertension chronotherapy—the judicious scheduling of conventional BP-lowering medications in accord with circadian rhythm determinants as a means of normalizing altered characteristics of the 24 h BP pattern, the most important ones being elevated asleep BP mean and attenuated sleep-time relative BP decline (percent decrease in mean BP during nighttime sleep relative to the mean BP during awake-time activity)—as a simple and cost-effective means of reducing CVD, stroke and other risk. These sensitive clinical biomarkers of such elevated risk can be determined only by around-the-clock ambulatory BP monitoring (ABPM), a diagnostic tool method that allows thorough description and quantification of all aspects of the BP nyctohemeral variation.18
Differential prognostic value of certain features of the 24 h bp pattern
Diagnosis of hypertension and clinical decisions regarding its treatment are based primarily on a limited number of daytime office BP measurements (OBPM), occasionally supplemented by wake-time patient self-assessments.19 However, numerous outcome trials and published meta-analyses substantiate that correlation between BP level and risk of target organ injury and CVD events is much higher for ABPM-derived parameters, particularly sleep-time BP, than it is for values derived from traditional daytime OBPM.20, 21, 22, 23, 24, 25, 26 Specific features of the 24 h BP pattern determined by ABPM have been extensively explored as biomarkers or mediators of target tissue injury and triggers of and risk factors for CVD (angina pectoris, myocardial infarction, cardiac arrest, sudden cardiac death, severe arrhythmias and pulmonary embolism) and cerebrovascular events (ischemic and hemorrhagic stroke).9, 27 The findings of multiple studies consistently document strong association between the abnormal physiologic feature of blunted sleep-time relative BP decline (nondipper/riser BP patterning) and increased incidence of fatal and nonfatal CVD events, not only in hypertensive20, 22, 23, 24, 28, 29, 30, 31, 32 but also normotensive individuals.33 Furthermore, various independent prospective studies demonstrate CVD events are better predicted by the asleep than awake or 24 h BP means.22, 25, 26, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40 As an illustrative example, a recent meta-analysis of 9 cohorts entailing 13 844 hypertensive patients concluded that, when analyzed individually, increases in clinic SBP as well as awake and asleep SBP means are all significantly associated with elevated CVD risk; however, when all three SBP measurements are simultaneously included into the survival model, only the asleep SBP mean prevails as independent predictor of CVD events.26
Overall, these and many other such prospective studies demonstrate elevated sleep-time BP constitutes a significant CVD risk factor, independent of daytime OBPM or ambulatory awake and 24 h BP means. Nonetheless, findings and conclusions of most previous ABPM studies may be imprecise because of inherent limitations of investigative methods,41, 42 leading to profound inaccuracies and inconsistencies between studies on the prognostic value of multiple ABPM-derived variables, particularly the awake and asleep SBP/DBP means. One major limitation of all previous studies addressing the merit of ABPM for predicting CVD risk—except the subsequently discussed MAPEC (Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares, that is, ambulatory blood pressure monitoring for prediction of cardiovascular events) study31, 32, 33, 37, 38, 39, 43, 44, 45, 46, 47, 48, 49—is reliance upon only a single, low-reproducible50 24 h ABPM evaluation per participant conducted at study inclusion. Such a study design is unsound because it presumes all features of the baseline-determined ambulatory BP pattern are maintained without alteration during the many years of follow-up, despite institution or modification of BP-lowering therapy, aging and/or development of target organ damage and concomitant morbidity.51 Lack of systematic and multiple ABPM evaluation of patients over time in long-term follow-up studies precludes the opportunity to explore potential reduction in CVD risk associated with modification of prognostic parameters by hypertension therapy, that is, either increase of sleep-time relative BP decline toward more normal dipper patterning or, more specifically, reduction of asleep BP mean.
The MAPEC study was designed as a prospective, randomized, open-label, blinded endpoint trial to test the hypothesis that bedtime hypertension chronotherapy entailing conventional hypertension medications exerts better ambulatory BP control plus improved CVD and stroke as well as new-onset type 2 diabetes and chronic kidney disease development and progression risk reduction than standard therapy, that is, all such prescribed hypertension medications ingested in the morning. Complete details of the rationale and design of the MAPEC study are reported elsewhere.31, 32, 33, 37, 38, 39, 43, 44, 45, 46, 47, 48, 49 Briefly, 3344 subjects (1718 male/1626 female) with baseline ABPM ranging from normotension to sustained hypertension were prospectively followed for a median duration of 5.6 years. Hypertensive participants at baseline were randomized to two treatment strategies: (1) all prescribed conventional hypertension medications ingested upon awakening or (2) complete daily dose of ⩾1 of them ingested at bedtime. At baseline and thereafter at yearly intervals (more frequently after doctor-ordered change in therapy—either to improve ambulatory BP control or avert sleep-time hypotension—aided by dedicated software for individualized ABPM evaluation52, 53), ambulatory BP and physical activity (wrist actigraphy to accurately derive the awake and asleep BP means on an individual basis54, 55) were simultaneously monitored for 48 consecutive hours.
In the MAPEC study, joint analysis of the multiple BP parameters potentially capable to contributing to CVD risk clearly substantiates that traditional OBPM does not independently predict CVD morbidity and mortality when the outcomes model is adjusted by the asleep BP mean (hazard ratio (HR)=1.44, 95% confidence interval (CI) 1.30–1.60, P<0.001, per s.d. elevation in asleep SBP mean; HR=1.09, 95% CI 0.97–1.23, P=0.123, per s.d. elevation in clinic SBP). The best Cox regression fully adjusted model includes only the asleep SBP mean (HR=1.43, 95% CI 1.29–1.58, P<0.001) and sleep-time relative SBP decline (HR=0.88, 95% CI 0.78–0.98, P=0.023). However, when the awake SBP mean is adjusted by the asleep SBP mean, only the later significantly predicts CVD outcomes (HR=1.67, 95% CI 1.45–1.92], P<0.001, per s.d. increase in asleep SBP mean; HR=0.88, 95% CI 0.75–1.02, P=0.094, per s.d. rise in awake SBP mean).31, 32
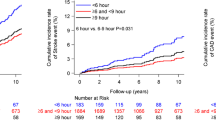
To further investigate the clinical relevance of the asleep BP mean on CVD risk, the studied population of the MAPEC study was divided into four groups according to BP level, that is, normal or elevated according to established ABPM thresholds of 135/85 mm Hg for the awake SBP/DBP means and of 120/70 mm Hg for the asleep SBP/DBP means,19, 18 independent of OBPM, at the final ABPM evaluation of the respective patients, that is, either before the documented CVD event in event subjects or latest assessment in nonevent individuals. The results shown in Figure 1 (top) indicate: (1) equivalent adjusted HR for total CVD events of participants with normal asleep BP mean whether awake BP mean is normal or elevated (P=0.489); (2) equivalent HR in hypertensive patients with elevated asleep BP mean, independent of awake BP mean (P=0.385); and (3) significantly higher adjusted HR of total CVD events in hypertensive patients with elevated than normal asleep BP mean, whether the awake BP mean is less than or greater than 135/85 mm Hg, the diagnostic thresholds that differentiate normotension from hypertension. Each of the four groups of participants categorized by awake and asleep SBP/DBP means (Figure 1, top) were further categorized according to either normal or elevated OBPM (using the currently accepted 140/90 mm Hg thresholds). Results of these analyses (Figure 1, bottom) denote CVD risk is significantly higher in the four groups classified by elevated asleep BP mean, regardless of whether the daytime OBPM or ABPM-derived awake BP mean is normal or elevated, than the other four groups of patients classified by normal sleep-time BP mean. In summary, the asleep BP mean, but not the OBPM or ABPM-derived awake BP mean, constitutes a highly significant independent prognosticator of CVD morbidity and mortality.31, 32
Adjusted hazard ratio (HR) of total cardiovascular disease (CVD) events in the MAPEC (Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares) study. (Top) Participants categorized into groups according to the level (normal or high) of ambulatory BP monitoring (ABPM)-derived awake and asleep systolic blood pressure (SBP) and diastolic blood pressure (DBP) means. (Bottom) Participants categorized into groups, both according to daytime office BP measurements (OBPM) level (normal or high) and ABPM-derived awake and asleep SBP and DBP means. OBPM-obtained SBP/DBP values were considered normal if <140/90 mm Hg and high otherwise. The awake SBP/DBP means were considered normal if <135/85 mm Hg and high otherwise. The asleep SBP/DBP means were considered normal if <120/70 mm Hg and high otherwise. Adjustments were applied for sex, age, type 2 diabetes, chronic kidney disease (CKD), sleep duration and hypertension treatment time—all medications upon awakening vs. full daily dose of ⩾1 medications at bedtime (updated from references 18, 37). A full color version of this figure is available at the Hypertension Research journal online.
Data from the MAPEC study, in which participants were repeatedly assessed by periodic 48 h ABPM, also permitted prospective evaluation of the impact of changes in OBPM and ABPM during follow-up on CVD risk. Progressive treatment-induced lowering of the awake, asleep and 48 h BP means, but not OBPM, when each is analyzed individually, is associated with significantly increased CVD event-free survival; however, reduction from baseline in the asleep SBP/DBP means is the most significant predictor of survival among all the tested ambulatory and OBPM parameters.31, 32 Changes due to altered health status or hypertension therapy during the 5.6-year median follow-up in morning BP surge, pre-awakening BP surge, and nighttime BP fall are not significantly associated with reduced or increased CVD risk and survival, but hypertension treatment-dependent progressive increase during follow-up in the sleep-time relative SBP decline toward the normal dipper pattern is significantly associated with reduced risk. Most important, when treatment-induced changes during follow-up in the asleep and awake BP means are entered jointly in the same time-dependent Cox regression model, progressive attenuation of the asleep SBP mean is significantly associated with increased event-free survival (adjusted HR=0.67, 95% CI 0.55–0.81, P<0.001, per s.d. reduction in asleep SBP mean), whereas progressive reduction in the awake SBP mean is not (adjusted HR=1.00, 95% CI 0.86–1.18, P=0.958, per s.d. decrease in awake SBP mean during follow-up). The best time-dependent Cox regression fully adjusted model includes only the progressive lessening of the asleep SBP mean (HR=0.76, 95% CI 0.68–0.85, P<0.001, per s.d. reduction in asleep SBP mean) and increase in the sleep-time relative SBP decline (HR=0.84, 95% CI 0.72–0.99, P=0.038, per s.d. increase in sleep-time relative SBP decline during follow-up). Therefore, a progressively diminished asleep BP mean, but not daytime OBPM or ABPM-derived awake BP mean, is a highly significant independent prognostic marker of reduced CVD morbidity and mortality risk; it therefore constitutes a novel therapeutic target for achieving increased CVD event-free survival.31, 32
Ingestion-time differences in PD of hypertension medications
The PK of hypertension medications are significantly influenced by the well-documented circadian rhythms in gastric pH, transport and emptying; gastrointestinal motility; biliary function; glomerular filtration; hepatic enzyme activity; and blood flow of the duodenum, liver and kidney.56, 57, 58 Statistically and clinically significant ingestion-time (more specifically circadian stage) differences in the PD, that is, therapeutic modulation of the features of the BP 24 h pattern and level and also risk to adverse effects, of hypertension therapies59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 can result not only from circadian rhythm dependencies of their PK but also their free fraction, degradation/clearance, receptor number/conformation and second messengers/signaling pathways of drug-targeted sites, for example, blood vessel, heart and kidney tissue and autonomic nervous system and RAAS.59, 62, 70 However, ingestion-time differences in the therapeutic and adverse effects of BP-reducing medications need not be solely dependent upon PK alone, as the timing of drug peak and trough blood concentrations (Cmax and Cmin) relative to the staging of the many underlying circadian rhythms that give rise to the unique BP 24 h pattern may be more important. Indeed, explanation of the enhanced BP regulation conveyed by bedtime vs. morning regimen of ACE inhibitors (ACEIs) and angiotensin-II receptor blockers (ARBs), as discussed subsequently, is theorized to result from drug Cmax or near Cmax coinciding in time to when the circadian rhythm-controlled RAAS activates, during the middle to late hours of nighttime sleep.59, 60, 61, 62, 63
Two common mistakes made in the design and conduct of chronopharmacology and chronotherapy trials of BP-lowering medications are: (1) failure to require as a key inclusion criterion that subjects have a life routine of diurnal activity alternating with nighttime sleep as confirmed by diary entries, and (2) selection of treatment times according to clock hour rather than biological markers of circadian stage, for example, upon morning awakening and at bedtime for participants adhering to a consistent diurnal wake and nocturnal sleep routine, to take into account individual differences in exact activity onset (awakening from repose) and activity offset (bedtime), as routinely done in our prospective trials.63 Another frequent error is reliance upon daytime OBPM, even if bolstered with self-assessed at-work and at-home wake-time BP data. The qualification of subjects for medication trials when relying alone on clinical cuff methods can be misleading because of incorporation of individuals with masked normotension (also termed isolated office hypertension, that is, elevated clinic BP but normal out-of-office BP determined by ABPM) and exclusion of otherwise high-risk patients with masked hypertension (normal OBPM and elevated ABPM, mainly during nighttime sleep).18, 37 Finally, reliance upon daytime clinic or home awake-time BP self-measurements do not enable evaluation of effects of different therapies and their dosing times on the pertinent characteristics of the 24 h BP pattern and level, such as the asleep SBP and DBP means and sleep-time SBP and DBP relative decline. Accordingly, the only valid means of researching and quantifying ingestion-time (circadian rhythm-dependent) differences in the PD effects of BP medications is through trials that incorporate 24 h or longer ABPM to enable reporting of therapeutic outcomes in terms of reductions of both the awake and asleep BP means individually derived by taking into consideration the actual activity and sleep spans of each participant. Thus, in this review of the current literature, we focus almost exclusively on trials that: (1) entail 24 h or longer patient ABPM and (2) report therapeutic effects of medication timings upon both actual patient awake and asleep SBP and DBP means, with the clock times of waking and asleep spans based upon actual diary or activity monitoring per participant as opposed to preassumed or software-generated default ones, which is too often the case in medications trials.
Numerous prospective randomized clinical trials clearly document that the magnitude of desired effects upon the 24 h BP pattern exerted by ACEIs, ARBs, calcium-channel blockers (CCBs), α-blockers, β-blockers and diuretics varies, often substantially, according to treatment time. Moreover, patient tolerance, in terms of adverse effects, to some classes of these therapies is greatly improved when ingested alone or in combination at bedtime than upon awakening from sleep.59, 60, 61, 62, 63 Table 1 reports, relative to baseline pretreatment values, the differential reduction of both awake and asleep SBP and DBP means, as well as differential effect upon their sleep-time relative decline, when hypertension medications of six different classes are routinely administered individually as a monotherapy or in combination upon awakening vs. at bedtime. The findings shown in this table were derived through PROBE (prospective randomized, open-label, blinded endpoint) trials that some of us conducted. In total, the trials entailed 2473 hypertensive patients adhering to a normal routine or daytime activity alternating with nighttime sleep who, both before and after treatment, were assessed simultaneously by 48 h ABPM at 20/30-min intervals and wrist actigraphy (recording of activity level at 1-min intervals) to enable accurate derivation of the awake and asleep SBP/DBP means54, 55 plus dipper/nondipper BP patterning. The contents of the table are consistent across the different classes of medications: bedtime, in comparison to morning, ingestion of hypertension therapy results in statistically and clinically significant enhancement of asleep BP mean reduction and also sleep-time relative BP decline and, therefore, improved normalization of the 24 h profile.
ACEI monotherapy
A substantial number of studies demonstrate that the ACEIs of benazepril, captopril, enalapril, imidapril, lisinopril, perindopril, quinapril, ramipril, spirapril, trandolapril and zofenopril, as a class and independent of medication terminal half-life, when routinely ingested in the evening or at bedtime in comparison with upon morning awakening: (1) exert significantly better BP-lowering effects upon the asleep than awake BP means (for extensive review see63), (3) better convert the daily BP profile toward or into the normal dipping one and/or (3) improve patient tolerance to therapy.59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69
This is exemplified by the findings of a clinical trial by Hermida et al.71 comprising 115 previously untreated hypertensive patients randomized to either upon-wakening or bedtime 5 mg ramipril monotherapy and who were assessed by 48 h ABPM before and after 6 weeks of treatment. Although ingestion time shows no differential effect on daytime OBPM or 48 h and awake SBP/DBP means, the bedtime compared with morning schedule causes very significant larger reduction of the asleep SBP/DBP means (−13.5/−11.5 vs. −4.5/−4.1 mm Hg; P<0.001 between groups; Table 1 and Figure 2, top). The differential ingestion time-dependent effects of ramipril on SBP are illustrated in the bottom panel of Figure 2. Ramipril reaches peak effect more rapidly when ingested at bedtime than upon morning awakening, giving rise to significantly greater therapeutic efficacy during the first 6 h following its ingestion. Moreover, duration of BP-lowering effect is shorter when ramipril is ingested upon awakening than at bedtime, resulting in BP reduction with bedtime ramipril administration being significantly greater during the last 12 h of the 24 h dosing interval.71
(Top) Changes from baseline (mm Hg) in office BP measurements (OBPM), 48 h awake (active span) and asleep (nighttime resting span) systolic blood pressure (SBP) means by 5 mg day−1 ramipril ingested upon awakening or at bedtime in patients with grade 1–2 essential hypertension assessed by 48 h ambulatory BP monitoring (ABPM) before and after 6 weeks of timed treatment. Probability values are shown for comparison of effects between the two treatment-time patient groups. (Bottom) Changes from baseline (mm Hg) during the 24 h in SBP after treatment with 5 mg day−1 ramipril ingested either upon awakening or at bedtime. *P<0.05 in BP reduction between the two treatment-time groups (updated from reference 71). A full color version of this figure is available at the Hypertension Research journal online.
The results of another study by some of us utilizing an identical investigative protocol and involving 165 previously untreated hypertensive patients demonstrate that the differential treatment-time effects of ACEIs upon the 24 h BP profile are independent of drug half-life. Treatment with the long (~40 h) terminal plasma half-life ACEI spirapril in a 6 mg once-daily dose for 12 weeks in patients randomized to bedtime rather than upon awakening dosing better decreases asleep SBP/DBP means (−12.8/−8.6 vs. −5.7/−4.6 mm Hg; P<0.001 between treatment time groups; Table 1 and Figure 3, top) and better enhances sleep-time relative BP decline toward normal dipper patterning.72 When ingested in the morning, spirapril evidences loss of its BP-lowering efficacy shortly after reaching peak effect, ~3 h after ingestion (Figure 3, bottom). In contrast, when ingested at bedtime, maximum BP-lowering effect is maintained for the ensuing 8 h span, thus showing greater efficacy, relative to morning administration, during this time interval. Thus, the efficacy of spirapril, like ramipril, is significantly greater during the last half, particularly the last 4 h, of the 24 h dosing interval with bedtime as compared with upon awakening administration (Figure 3, bottom).72 The findings for zofenopril (30 mg once daily for 1 month) for 33 previously untreated hypertensive patients are similar to those for ramipril and spirapril (Figures 2 and 3): bedtime compared with awakening dosing of zofenopril better reduces asleep BP mean and increases the proportion of patients with controlled ambulatory BP from 51.5 to 84.8% (P<0.001).73
(Top) Changes from baseline (mm Hg) in office BP measurements (OBPM), 48 h awake (active span) and asleep (nighttime resting span) systolic blood pressure (SBP) means by 6 mg day−1 spirapril ingested upon awakening or at bedtime in patients with grade 1–2 essential hypertension assessed by 48 h ABPM before and after 12 weeks of timed treatment. Probability values are shown for comparison of effects between the two treatment-time patient groups. (Bottom) Changes from baseline (mm Hg) during the 24 h in SBP after treatment with 6 mg day−1 spirapril ingested either upon awakening or at bedtime. *P<0.05 in BP reduction between the two treatment-time groups (updated from reference 72). A full color version of this figure is available at the Hypertension Research journal online.
A troublesome adverse effect of ACEIs that often compromises compliance is persistent dry cough that, for example, in enalapril-treated patients shows a prevalence of ~12%.74 This adverse effect is triggered by ACEI-induced bradykinin synthesis; however, as demonstrated for enalapril,75, 76 switching its ingestion time from morning (1000 h) to evening (2200 h) minimizes or averts excessive bradykinin synthesis and risk of dry cough in patients adhering to a diurnal activity/nighttime sleep routine. Moreover, BP reduction is still significantly reduced 24 h after taking enalapril at night, but not after taking it in the morning, indicating prolonged BP-lowering action of enalapril is possible only with evening administration.
ARB monotherapy
Prospective clinical trials with irbesartan, olmesartan, telmisartan and valsartan validate that significant ingestion-time differences in therapeutic effects are independent of drug terminal half-life.59, 60, 61, 62, 63 As found for ACEIs, choice of treatment time with the various ARBs exerts no differential reduction of the awake SBP/DBP means; however, reduction of the asleep SBP/DBP means is more profound by the bedtime than morning regimen, thereby significantly decreasing prevalence from baseline of nondipping.77, 78, 79, 80, 81, 82, 83, 84 Importantly, bedtime but not morning ingestion of valsartan,80 candesartan85, 86 and olmesartan87 also significantly lessens urinary albumin excretion, a measure of renal hemodynamics and pathology that correlates strongly with extent of decrease in asleep BP mean and increase in sleep-time relative BP decline,80 and in one study improvement of baroreflex sensitivity.86
Hermida et al.80 first assessed the efficacy of valsartan monotherapy (160 mg once daily for 12 weeks) when ingested either upon awakening or at bedtime by 90 hypertensive patients. When valsartan is ingested at bedtime, attenuation of asleep SBP/DBP means is significantly greater than that of awake SBP/DBP means (respectively, −17.9/−13.3 vs. −12.0/−9.8 mm Hg; P=0.009/0.015). Consequently, the bedtime schedule results in a highly significant average increase by 6% in sleep-time relative BP decline that translates into 73% reduction from baseline in the number of nondipper patients.80 These results are corroborated by two subsequent independent prospective trials, the first conducted on elderly hypertensive patients,81 who as a group are characterized by greater blunting of sleep-time relative BP decline than younger hypertensive patients,51 and the second conducted on nondipper hypertensive patients.79, 82 For the composite of all these trials entailing valsartan, reduction of asleep SBP/DBP mean is significantly greater when this ARB is ingested at bedtime than upon awakening (respectively, −18.4/−12.6 vs. −12.4/−8.9 mm Hg; P<0.001 between treatment time groups; Figure 4, top). The differential effects of 160 mg day−1 valsartan on SBP as a function of the time of drug ingestion are shown in Figure 4 (bottom). Significant lowering of SBP during the entire 24 h dosing interval is achieved independent of medication ingestion time. However, SBP reduction is significantly greater during the first 10 h following treatment when valsartan is ingested at bedtime. The greater reduction of asleep SBP/DBP means by a bedtime than morning schedule with a higher dose of 320 mg day−1 valsartan is also documented in a recent investigator-promoted independent study on hypertensive patients with sleep apnea,88 but not in an industry-promoted trial limited by faulty study design, that is, selection of treatment times according to clock hour plus improper reliance upon daytime and nighttime BP means, instead of the most pertinent awake and asleep ones, according to an identical fixed clock-hour span for all participants.89
(Top) Changes from baseline (mm Hg) in office BP measurements (OBPM), 48 h awake (active span) and asleep (nighttime resting span) systolic blood pressure (SBP) means by 160 mg day−1 valsartan ingested upon awakening or at bedtime in patients with grade 1–2 essential hypertension assessed by 48 h ambulatory BP monitoring (ABPM) before and after 12 weeks of timed treatment. Probability values are shown for comparison of effects between the two treatment-time patient groups. (Bottom) Changes from baseline (mm Hg) during the 24 h in SBP after treatment with 160 mg day−1 valsartan ingested either upon awakening or at bedtime. *P<0.05 in BP reduction between the two treatment-time groups. A full color version of this figure is available at the Hypertension Research journal online.
Of particular interest is the fact that telmisartan, even though having a terminal plasma half-life of >24 h,90 exerts significant ingestion-time differences in therapeutic effects.83 A total of 215 grade 1–2 essential hypertensive patients were randomized to 12 weeks of once-daily 80 mg telmisartan monotherapy scheduled either consistently upon awakening or at bedtime. Significant and comparable lowering of OBPM and the ABPM-derived 48 h and awake SBP/DBP means from baseline is achieved by the two treatment strategies (Table 1 and Figure 5, top). However, bedtime, relative to morning, treatment achieves significantly greater attenuation of asleep SBP/DBP means (−13.8/−9.7 vs. −8.3/−6.4 mm Hg; P<0.001 between treatment time groups; Table 1 and Figure 5, top). Thus, when telmisartan is ingested upon awakening, the sleep-time relative SBP/DBP decline is actually slightly decreased toward the nondipping pattern (−1.6/−1.0; P=0.010/0.157), whereas when ingested at bedtime it is significantly enhanced toward normal (3.1/3.9; P<0.001), thereby reducing prevalence from baseline of the abnormal nondipper BP pattern by 76% (P<0.001).83 The differential effects of telmisartan on ambulatory BP relative to its administration time are shown in Figure 5 (bottom). Despite its long half-life, when telmisartan is ingested in the morning upon awakening, rather than at bedtime, it progressively loses BP-lowering efficacy 16 h after ingestion. Consequently, BP reduction is significantly greater during the last 8 h of the dosing interval when the medication was ingested at bedtime. In addition, treatment efficacy on BP is significantly greater during the first 2–8 h after bedtime dosing (Figure 5, bottom). These differential ingestion time effects can be largely explained in terms of when, during the 24 h, highest and lowest drug concentrations are attained relative to the activation of the circadian rhythm in the RAAS, that is, during nighttime sleep. The same explanation applies to the differential effects of ACEIs when ingested in the morning upon awakening vs. at bedtime, as discussed in the previous section.
(Top) Changes from baseline (mm Hg) in office BP measurements (OBPM), 48 h awake (active span) and asleep (nighttime resting span) systolic blood pressure (SBP) means by 80 mg day−1 telmisartan ingested upon awakening or at bedtime in patients with grade 1–2 essential hypertension assessed by 48 h ambulatory BP monitoring (ABPM) before and after 12 weeks of timed treatment. Probability values are shown for comparison of effects between the two treatment-time patient groups. (Bottom) Changes from baseline (mm Hg) during the 24 h in SBP after treatment with 80 mg day−1 telmisartan ingested either upon awakening or at bedtime. *P<0.05 in BP reduction between the two treatment-time groups (updated from reference 83). A full color version of this figure is available at the Hypertension Research journal online.
Other hypertension monotherapies
Prospective clinical trials involving amlodipine, cilnidipine, diltiazem, isradipine, nifedipine, nisoldipine and nitrendipine indicate that dihydropyridine CCBs generally reduce BP homogeneously throughout the 24 h, whether routinely ingested in the morning or evening.63 However, dosing time can be a determinant of risk for drug-associated adverse effects. The findings of a randomized by treatment time study by Hermida et al.91, 92 of 238 previously untreated hypertensive patients reveal that 8-week bedtime as compared with upon awakening 30 mg once-daily nifedipine GITS (gastrointestinal therapeutic system) monotherapy significantly reduces incidence of peripheral edema from 13 to 1% (P<0.001).
Other hypertension monotherapies, including α-blocker doxazosin,93 β-blockers carvedilol94 and nebivolol95 and loop-diuretic torasemide,96 also evidence significant ingestion-time differences in beneficial effect (Table 1). All these medications exert enhanced asleep BP reduction and longer duration of BP-lowering effect when consistently ingested at bedtime than upon morning awakening.
Fixed combination hypertension therapy
Most hypertensive patients require treatment with more than one BP-lowering medication to achieve target BP goals.19 Despite substantial evidence of ingestion-time differences in effects of various classes of hypertension monotherapies, thus far only a limited number of studies have entailed trialing of combination medications at different times of the day.63
Middeke et al.97 reported that once-daily 25 mg captopril/12.5 mg hydrochlorothiazide combination therapy (13 hypertensive men for 3 weeks) is slightly more effective in reducing nighttime BP mean when ingested at 2000 h and significantly more effective (P<0.01) in reducing daytime BP when taken at 0800 h. Meng et al.98 randomized 40 hypertensive patients whose BP was uncontrolled with either amlodipine or fosinopril monotherapy into two groups for 4 weeks of combination therapy with both medications. Those of group A routinely took 5 mg amlodipine in the morning (0700–0800 h) and 10 mg fosinopril at bedtime, and those of group B always took the two medications together consistently in the morning (0700–0800 h). The nighttime SBP/DBP means of group A patients show substantial reduction, by almost threefold greater amount, than group B patients (−22.4/−17.4 vs. −7.6/−6.3 mm Hg, P<0.001). Furthermore, the sleep-time relative BP decline is increased in group A and decreased in group B participants, thereby converting the 24 h profile of group A but not group B participants toward normal dipper patterning. Zeng et al.99 examined the differential therapeutic effects of 12 weeks of morning (0800 h) vs. evening (2200 h) fixed-dose 5 mg amlodipine/25 mg hydrochlorothiazide single-pill combination therapy in 80 hypertensive patients. Evening, compared with morning, treatment significantly better reduces the nighttime BP mean, resulting in threefold lesser proportion of patients with nondipper BP patterning (25% vs. 8%; P<0.001). Hoshino et al.,87 in a small sample study of 31 hypertensive patients, compared the BP-lowering effect of bedtime vs. morning amlodipine/olmesartan combination therapy, with titration of doses on a per-patient basis ranging from 2.5 to 10 mg for amlodipine and 20 to 40 mg for olmesartan. The bedtime relative to morning regimen more effectively reduces nighttime BP in nondipper but not dipper patients, and it more effectively reduces urinary albumin/creatinine ratio (42.5±59.9 vs. 75.3±26.4 mg g−1; P=0.044), inferring bedtime scheduling of this combination therapy exerts significantly better renoprotection.
In a much larger and detailed combination therapy study, Hermida et al.100 randomly assigned 203 hypertensive patients to four different 160 mg valsartan/5 mg amlodipine regimens for 12 weeks: both medications ingested together upon awakening, both ingested together at bedtime or either one of them ingested upon awakening and the other at bedtime. Taking both medications together at bedtime best reduces asleep SBP/DBP means, thereby significantly increasing sleep-time relative BP decline toward more normal dipper patterning (P<0.001; Table 1). Finally, Hermida et al.101 evaluated 204 hypertensive patients with uncontrolled ambulatory BP according to published ABPM criteria18, 19 after initial randomization to valsartan monotherapy (160 mg once daily for 12 weeks) either upon awakening or at bedtime. Hydrochlorothiazide (12.5 mg) was added and administered as a single-pill combination formulation with valsartan, with the patients adhering to the same original awakening or bedtime treatment time schedule for an additional 12 weeks. Bedtime relative to upon awakening combination therapy better reduces asleep means of SBP (20.1 vs. 16.0 mm Hg, P=0.015; Table 1) and pulse pressure, that is, SBP/DBP, a measure of the compliance of the arterial tree (6.5 vs. 4.0 mm Hg, P=0.007), and significantly reduces nondipper BP patterning from 59% of patients at baseline to 23% at study conclusion (P<0.001).
Bedtime chronotherapy with conventional medications in difficult to control and complicated hypertension
Resistant hypertension (RH)
RH constitutes a clear illustration of the clinical relevance of a chronotherapeutic strategy that takes into account circadian changes in the physiology and biochemistry of BP control and regulation. RH patients are at a considerably greater risk for renal disease and insufficiency and CVD and stroke events than are patients whose BP is well controlled by medication.39, 102 By definition, hypertension is designated as resistant to treatment when lifestyle measures plus therapeutic doses of ⩾3 prescription BP-lowering therapies, one preferably being a diuretic unless contraindicated, fails to reduce SBP and DBP to clinical cuff BP threshold values,102 a definition we contend lacks validity,18 as later discussed. Current clinical strategies for RH, which are far too often unsuccessful, entail either prescription of additional medications or exchange of ⩾1 of them in an attempt to achieve improved synergic BP-reducing effects.19, 102 Based upon the information we presented in the preceding sections, which clearly substantiates enhancement of BP-lowering efficacy by the bedtime treatment schedule, it is logical to question whether RH patients are said to be ‘resistant’ to therapy because it is prescribed and ingested at the wrong, morning, rather than right, bedtime (that is, circadian stage) when most effective.63, 65, 103, 104, 105, 106, 107
One cross-sectional study conducted by Hermida et al.104 entailing 700 RH patients who were assessed by 48 h ABPM reports the proportion of patients with controlled ambulatory BP (awake and asleep SBP/DBP means below current diagnostic thresholds) is twofold greater in those who ingest the entire daily dose of ⩾1 hypertension medications routinely at bedtime than in those who ingest all medications upon awakening. In addition, the prevalence of nondipping is significantly lower in patients taking ⩾1 medications at bedtime than in those taking all of them on awakening (57% vs. 82%).104 A larger identically designed cross-sectional study of 1794 RH patients evaluated by 48 h ABPM also documents the proportion of patients showing control of ambulatory BP among those ingesting the entire daily dose of ⩾1 medications at bedtime is significantly higher (31.9% of patients) than among those ingesting all medications upon awakening (23.1%; P<0.001).106 Moreover, bedtime, vs. upon awakening, treatment better lowers asleep SBP/DBP means (by 9.7/4.4 mm Hg, P<0.001), resulting in significantly greater (by 5.8%; P<0.001) sleep-time relative BP decline and thus significantly lower prevalence of nondipper BP patterning (40% vs. 83%, respectively; P<0.001).
Another even larger trial by Hermida et al.,65 also entailing 48 h ABPM, assessed role of treatment-time regimen on 24 h BP patterning of 2899 RH patients enrolled in the ongoing multicenter Hygia project. The Hygia project, comprising patients of primary care centers of Galicia (Northwest Spain), is a prospective investigation of the prognostic value of ABPM and treatment-time strategy on BP control and CVD risk. ABPM is done upon recruitment and at least annually thereafter, always for 48 h and with diary recording of time of retiring to bed at night and awakening in the morning to achieve confidence of findings and accurately derive awake and asleep SBP and DBP means. Of the total cohort of 2899 RH patients, 1084 consistently took all hypertension medications upon awakening (awakening regimen), 1436 others took the entire daily dose of ⩾1 of them at bedtime (bedtime chronotherapy regimen) and the remaining 379 patients took split equal doses of ⩾1 medications twice daily, upon awakening and at bedtime (b.i.d. regimen). The bedtime chronotherapy regimen, as compared with the awakening and b.i.d. ones, achieves significantly higher prevalence of properly controlled ambulatory BP, best lowers asleep SBP/DBP means and best enhances sleep-time relative BP decline, resulting in lowest prevalence of nondipping (54.4% vs. 80.5% and 77.3%, respectively; P<0.001 among the three treatment groups).65
The findings and conclusions of these cross-sectional RH studies were prospectively validated in a randomized trial that examined the role of treatment time, without increasing number of prescribed medications, on ambulatory BP pattern and control of 250 true RH patients, defined by uncontrolled awake and/or asleep ambulatory BP means when ingesting all three prescribed BP-lowering medications upon awakening.105 Participants were randomly assigned to one of two groups according to the designated modification of treatment strategy: (1) group A: exchange of 1 of the 3 medications for a new one, and retaining the same upon-awakening ingestion schedule; and (2) group B: also exchange of 1 of the 3 medications for a new one, but always ingesting it at bedtime. The 48 h ABPM studies conducted before and after 12 weeks of the new therapeutic schemes reveals for group A no change in ambulatory BP from baseline and slight increase in nondipping prevalence from 79% at baseline to 86% at study conclusion (P=0.131), and for group B significant reduction in ambulatory 48 h SBP/DBP means (−9.4/−6.0 mm Hg; P<0.001), with greater decrease of asleep than awake BP, and hence the proportion of patients displaying dipper patterning increases from only 16% at baseline to 57% at study conclusion (P<0.001).105 Finally, the findings of a recent small study of 27 RH patients by another group108 are consistent with those of the above cited studies. Shifting all nondiuretic hypertension medications from morning to evening not only significantly reduces nighttime BP (P=0.005) but enhances sleep-time relative BP decline towards more dipper patterning.
Most important, among the RH patients randomized according to time of treatment in the MAPEC study, those who routinely ingested the entire daily dose of ⩾1 hypertension medications at bedtime showed significantly lower HR of total CVD events (adjusted for the variables of age, sex and diabetes) than those who ingested all their medications upon awakening (0.38, 95% CI 0.27–0.55, P<0.001).39 The difference between treatment-time groups in the adjusted HR of major events (a composite of CVD death, myocardial infarction, ischemic stroke and hemorrhagic stroke) is also statistically significant (0.35, 95% CI 0.18–0.68, P=0.002). Based upon the currently available scientific evidence, bedtime ingestion of the complete daily dose of ⩾1 BP-lowering medications constitutes the most cost-effective therapeutic approach yet of improving both BP control and CVD event-free survival of patients who when managed by a morning treatment approach are incorrectly said to be inherently, that is, physiologically, resistant to BP control.39
Taken together, the findings of all the above reviewed studies verify a bedtime hypertension therapy regimen that entails the full daily dose of ⩾1 medication for RH, which is properly established by around-the-clock ABPM, is the therapeutic scheme of choice.18, 109 The collective findings additionally indicate the current definition of RH is invalid and must be modified to take into account the major deterministic variable of treatment time; accordingly, a patient should be categorized as resistant to treatment only if his/her ABPM-determined awake and/or (preferably) asleep SBP/DBP means are greater than the reference diagnostic thresholds18, 19 when at least one of the prescribed ⩾3 hypertension medications of different classes, ideally including a diuretic unless contraindicated, is ingested in full daily dose at bedtime.18
Chronic kidney disease (CKD)
Prevalence of hypertension is elevated in CKD, increasing with diminishing estimated glomerular filtration rate, and being as high as 86% in end-stage renal disease according to one report.110 Elevated asleep BP (sleep-time hypertension) and nondipper BP patterning are also prevalent in CKD.111, 112, 113, 114, 115, 116 Mojón et al.116 utilizing 48 h ABPM to assess 10 271 hypertensive participants enrolled in the ongoing hypertension treatment time outcomes Hygia Project report that: (1) prevalence of nondipper BP patterning is much greater in CKD than in the absence of CKD (60.6% vs. 43.2%, respectively; P<0.001 between groups); (2) prevalence of riser BP patterning (sleep-time relative SBP decline <0) is more than twofold larger in CKD than in the absence of CKD (17.6% vs. 7.1%, respectively; P<0.001) and (3) perhaps most importantly, among CKD participants with uncontrolled BP, 90.7% evidence sleep-time hypertension. These collective findings motivate testing of chronotherapeutic interventions for CKD, as reviewed below, to improve management of high BP, curtail disease progression and reduce CVD and stroke risk of these exceedingly vulnerable patients.
The impact of treatment-time regimen on ambulatory BP patterning and control in CKD, defined according to current recommendations,117 that is, estimated glomerular filtration rate <60 ml min−1 per 1.73 m2 or albuminuria (albumin/creatinine ratio ⩾30 mg gCr−1), or both, at least twice within a 3-month span, was recently investigated by Crespo et al.115 Among the 2659 evaluated hypertensive participants of the Hygia Project with CKD, 1446 ingested all BP-lowering medications upon awakening and 1213 others ingested the entire daily dose of ⩾1 of them at bedtime. Among the latter, 359 patients took all such medications at bedtime, whereas 854 ingested the complete daily dose of some of them upon awakening and the rest of them at bedtime. Patients managed with either one of the bedtime chronotherapy regimens, relative to those managed with the conventional upon awakening one, evidence significantly better: (1) reduction of asleep SBP/DBP means, (2) enhancement of sleep-time relative BP decline (P<0.001) and (3) attenuation of nondipping prevalence (68.3% in those managed conventionally vs. 54.2% and 47.9%, respectively, in those managed by the ⩾1 and all-medications bedtime chronotherapy regimens; P<0.001 between groups). Moreover, prevalence of riser BP patterning is significantly lower in participants ingesting ⩾1 (15.7%) or all (10.6%) hypertension medications at bedtime rather than all of them upon awakening (21.5%; P<0.001 between groups). Finally, patients ingesting all their medications at bedtime show significantly higher prevalence of controlled ambulatory BP (P<0.001) that is achieved by significantly fewer BP-lowering medications (P<0.001) compared with patients of the other treatment cohorts and who evidence inferior BP control.115
Minutolo et al.,118 who evaluated a rather small sample of 32 uncontrolled nondipper CKD patients, report significant reduction of the nighttime BP mean, with consequent decreased urinary albumin excretion, after shifting 1 BP-lowering medication from morning to evening. Another study entailing 151 black participants enrolled in the African American Study of Kidney Disease with controlled clinic and awake BP somewhat differs.119 This study compared the effects on nocturnal SBP (improperly defined according to an identical fixed clock-hour span across all participants) of either shifting to bedtime of an already prescribed once-a-day hypertension medication or adding at bedtime a new low-dose one. Both strategies decrease nocturnal SBP, but not significantly (P=0.08), prompting the authors to conclude bedtime chronotherapy might be of limited advantage in reducing nighttime BP in hypertensive African-American patients and/or CKD. However, as earlier discussed, failure to detect statistical significance could be because of faulty study design, that is, failure to assess changes in the actual asleep SBP mean based on data truly representative of the sleep period of each participant rather than an assumed, and most likely poorly representative, common nighttime span defined by a priori selected clock-time references. Indeed, confirmation of the expected better effect of bedtime chronotherapy, relative to conventional morning time therapy, for black African individuals is reported in a Nigerian study of 165 high-BP presumably diurnally active patients randomized to 12 weeks of either morning (1000 h) or evening (2200 h) hypertension treatment. The bedtime therapeutic strategy, in comparison with the conventional morning one, results in significantly greater reduction not only of BP but also of left ventricular mass (P<0.001).120 Finally, a recent study of 60 nondipper Chinese CKD patients asserts that bedtime relative to morning time valsartan (with doses ranging per patient from 80 to 320 mg) therapy for 1 year achieves significantly greater reduction of not only nighttime BP and left ventricular mass, as in the Nigeria study,120 but also albuminuria (P always <0.05).121 Because of the very high prevalence of abnormal 24 h BP profiling in CKD, that is, sleep-time hypertension and nondipping and rising BP patterning, plus documented better effects of a bedtime hypertension regimen on asleep BP regulation,59, 60, 61, 62, 63 as documented in the several above reviewed studies, bedtime treatment is now recommended as the preferred strategy to manage hypertension in CKD patients.18, 41, 45, 64, 68
Diabetes
There is strong association between diabetes and elevated risk of end-organ damage, stroke and CVD morbidity and mortality. In addition, nondipping and sleep-time hypertension are highly prevalent in patients with diabetes.122, 123, 124, 125 Ayala et al.124 compared features of the ambulatory BP pattern of 2954 hypertensive patients with type 2 diabetes and 9811 hypertensives without diabetes enrolled in the Hygia Project. Prevalence of nondipping is significantly higher in those with than without diabetes (62.1% vs. 45.9%; P<0.001); however, prevalence of riser BP patterning constitutes the most profound difference between groups (19.9% vs. 8.1% in patients with and without diabetes, respectively; P<0.001). In addition, 89.2% of uncontrolled hypertensive patients with diabetes in this cohort evidence sleep-time hypertension. Despite the very high prevalence of sleep-time hypertension and nondipper and riser patterns, only a small number of investigations have explored the impact of hypertension treatment time on BP regulation and control in individuals with diabetes.
A crossover design investigation by Tofé and García126 evaluated ambulatory BP response of 40 hypertensive patients with type 2 diabetes to olmesartan (40 mg once daily for 8 weeks) when ingested either upon awakening or at bedtime. They found bedtime treatment results in both significantly greater reduction of nighttime SBP (−16.2 vs. −11.8; P=0.007) and increase in sleep-time relative SBP decline (7.4 vs. 2.2%; P<0.001). Rossen et al.127 also used a crossover design to investigate the effect of change of ingestion time of hypertension medications in 41 patients with type 2 diabetes, finding that bedtime in comparison with morning ingestion of BP-lowering drugs results in significant reduction of nighttime SBP mean (7.5 mm Hg, P<0.001) with nonsignificant reduction of daytime SBP mean (1.3 mm Hg, P=0.336). Moyá et al.125 evaluated the differential beneficial effects of hypertension treatment-time regimen on ambulatory BP patterning of 2429 hypertensive participants of the Hygia Project with type 2 diabetes. Among them, 1176 ingested all BP-lowering medications upon awakening and 1253 the entire daily dose of ⩾1 medications at bedtime—336 patients taking all hypertension medications at bedtime, and 917 the entire daily dose of some of them upon awakening and others at bedtime. Ingestion of ⩾1 medications at bedtime, as compared with ingestion of all medications upon awakening, significantly better reduces asleep SBP/DBP means and better normalizes sleep-time relative BP decline. Thus, prevalence of nondipping is significantly higher when all hypertension medications are taken upon awakening (68.6%) than when ⩾1 (55.8%) or all of them (49.7%; P<0.001 between groups) are ingested at bedtime. The latter treatment group, relative to all the other ones, also shows significantly higher prevalence of properly controlled ambulatory BP (P<0.001) that is achieved by a significantly lesser number of hypertension medications (P<0.001).125
Finally, Suzuki and Aizawa128 provide an example of the misleading conclusions that commonly result from poorly conceptualized protocols when applied to assessing chronotherapeutic trials. These investigators randomized 34 already treated hypertensive patients with type 2 diabetes into three valsartan (160 mg) therapy groups defined by the ingestion of: the entire daily dose following breakfast or dinner, or b.i.d., that is, one-half the daily dose (80 mg) in the morning and other half (80 mg) in the evening. The investigators report no significant between-group difference in reduction of OBPM or home self-measured BP. Findings of this and several other studies of administration time differences in therapeutic effect of hypertension medications that rely upon daytime OBPM and/or home BP measurements alone are of little, if any, practical utility for many reasons. First, ‘white-coat’ and ‘masking’ effects may compromise representativeness of OBPM, whereas inconsistent technique of self-assessment and poor patient compliance may compromise accuracy of home BP data. Second, choice of dosing times is based on clock time rather than biological time relative to the individual patient sleep–wake routine. Third, and most important, the investigative protocol of these studies cannot collect data throughout the entire 24 h to reliably derive clinically meaningful characteristics of the daily BP profile closely linked with CVD risk, that is, asleep SBP mean and sleep-time relative SBP decline. Thus, the findings of improperly conceptualized protocols, as exemplified by this investigation, are not only useless but misleading by adding confusion, uncertainty and unfounded controversy to the medical literature and patient care.
Nondipper hypertension
Patients diagnosed with RH,65, 104, 105, 106, 107, 108 CKD115, 118, 119, 121 and diabetes125, 126, 127 have high prevalence of nondipping, as reviewed in preceding sections. Several chronotherapy trials specifically researched control of asleep BP and/or increase of sleep-time relative BP decline (dipping) of nondipper hypertensive patients in the absence of such diagnoses.
The first such bedtime chronotherapy trial entailing nondipping hypertensive patients was conducted by Hermida et al.79 It involved 148 enrollees of the MAPEC study randomized either to 160 mg valsartan upon awakening or at bedtime who were assessed by 48 h ABPM before and after 12 weeks of timed therapy. They reported significant substantial lowering from baseline of the 48 h SBP/DBP means (P<0.001) is ingestion-time independent (upon awakening treatment: −13.1/−8.4 vs. at bedtime treatment: −14.6/−10.1 mm Hg; P>0.126 for treatment-time effect). However, they found reduction of asleep SBP/DBP means to be significantly greater when valsartan is consistently taken at bedtime than conventionally upon awakening (respectively, −21.1/−13.9 vs. −12.5/−8.3 mm Hg; P<0.001 between treatment-time groups), resulting in significant enhancement of sleep-time relative BP decline and 75% of the patients reverting to normal dipper BP patterning. In addition, the bedtime chronotherapy not only significantly increases the proportion of patients with controlled ambulatory BP but significantly decreases urinary albumin excretion.80 An extension of this valsartan trial to include a total of 200 nondipper hypertensive patients yielded similar favorable results of the bedtime chronotherapy strategy relative to the traditional morning one, that is, more aggressive reduction of asleep BP mean plus improved normalization of sleep-time relative BP decline.82
A prospective, double-blind, placebo-controlled study by Qiu et al.129 involving 121 treated nondipper hypertensive patients randomized to evening (2200 h) 12.5 mg captopril or placebo treatment indicates this ACEI treatment strategy both significantly reduces nighttime BP and restores normal BP dipping in 70% of patients. The study, however, lacks a morning-time treatment comparison group to enable proper evaluation of findings. Another trial by Takeda et al.130 entailed 71 Japanese hypertensive patients, approximately half of them being nondippers, who had been ingesting long-acting once-daily BP-lowering medications in the morning. Shifting therapy for the 35 nondipper patients from morning to bedtime results in slight increase of daytime SBP/DBP means (+5/+3 mm Hg; P<0.02) and marked decrease in nighttime ones (−13/−6 mm Hg, P<0.001), thereby enhancing sleep-time relative SBP decline from 2.6 to 15.5% (P<0.001). Finally, Farah et al.131 investigated the role of treatment time on BP patterning of 60 nondipper hypertensive patients randomly assigned to continue ingestion of their prescribed BP-lowering medications upon awakening or shift in the ingestion of all of them to bedtime. Investigators verified significant reduction in nighttime BP mean among patients transferred to the bedtime therapy schedule, with 86% of them showing controlled ambulatory BP.
Influence of bedtime hypertension chronotherapy on the risk of CVD and stroke events
The published clinical trials reviewed in the preceding sections substantiate advantage of bedtime chronotherapy, as compared with the traditional morning time, strategy of dosing conventional hypertension medications to regulate asleep SBP/DBP and normalize the 24 h BP profile, even of difficult to control RH, CKD, type 2 diabetic and nondipper patients. This section, based on review of the findings of long-term outcomes trials, addresses the question of whether the bedtime hypertension approach is more protective against nonfatal and fatal stroke and CVD events than the traditional morning one.
The Heart Outcomes Prevention Evaluation (HOPE) trial tested the hypothesis that adding the ACEI ramipril vs. placebo to already existing BP-lowering, cholesterol-reducing and other preventive strategies significantly reduces CVD and stroke events in a cohort of 9297 high-risk CVD patients ⩾55 years of age.132 The results of the HOPE trial establish that add-on bedtime ramipril relative to placebo therapy significantly reduces the primary outcome variables of death from CVD causes and new-onset myocardial infarction and stroke plus secondary ones of death from any cause, revascularization procedures, cardiac events, complications of diabetes and hospitalizations for heart failure. Nonetheless, the HOPE trial protocol does not include a comparator morning ramipril treatment arm; thus, it does not enable testing the hypothesis that bedtime hypertension chronotherapy with ⩾1 conventional medications best reduces CVD and stroke risk.
The Syst-Eur trial investigated whether evening dihydropyridine CCB nitrendipine therapy, compared with placebo, reduces stroke and other CVD complications in 4695 elderly patients with isolated systolic hypertension diagnosed by OBPM.133 The assumed rationale for the chosen treatment regimen apparently is expected reduced risk of drug-induced peripheral edema and associated patient discontinuations with evening vs. morning CCB dosing, an assumption verified by Hermida et al.92 through study of nifedipine GITS some years later. After 2 years of follow-up, the investigators reported active treatment reduces the primary endpoint of stroke by 42% (P=0.003), CVD mortality by 27% (P=0.07) and total CVD outcomes by 31% (P<0.001).129 The almost identical protocol of the Syst-China trial involving 2394 patients found after 3 years of follow-up that active treatment reduces total stroke events by 38% (P=0.01), total mortality by 39% (P=0.003), CVD mortality by 39% (P=0.003), stroke mortality by 58% (P=0.02) and total CVD outcomes by 37% (P=0.004).134 Similar to the HOPE trial, the Syst-Eur and Syst-China trials were not designed to assess comparative effects of morning-time treatment on CVD and stroke risk.
The Controlled Onset Verapamil Investigation of Cardiovascular Endpoints (CONVINCE) trial was designed to determine whether initial treatment with 180 mg of the unique COER (controlled-onset extended-release) verapamil formulation, specifically designed for bedtime ingestion so as to achieve peak drug concentrations upon morning arising and initial hours of the diurnal activity span when BP is assumed to rapidly rise from its lowest (sleep-time) level, is equivalent to morning treatment with either 50 mg of the β-agonist atenolol or 12.5 mg of the diuretic hydrochlorothiazide in preventing as primary outcomes myocardial infarction, stroke or CVD death.135 Bedtime ingestion of COER verapamil significantly reduces morning BP but it exerts only a limited effect on asleep BP, as documented in one randomized trial showing twofold greater reduction in the awake than asleep SBP/DBP means.136 The CONVINCE trial was terminated prematurely because the sponsoring pharmaceutical company, for commercial reasons, closed the trial 2 years earlier than planned; thus, the median follow-up of participants was only 3 years. At the end of the abbreviated 3-year follow-up period, there were no differences in primary outcome events between the two tested treatment strategies. Thus, the CONVINCE trial failed to substantiate protection against any of the CVD outcome variables theorized to result through specific attenuation of the rapid rise and level of morning BP. An unrecognized undesired consequence of the COER verapamil bedtime treatment strategy is increased prevalence of the higher CVD risk nondipper BP patterning as a consequence of strong BP-lowering effect on wake-time BP but weak effect on sleep-time BP.
The tested hypertension medications of ramipril, nitrendipine and COER verapamil of the above reviewed investigations concerning evening therapeutic strategies do not include a comparator awakening treatment-time arm; thus, they are not valid chronotherapy outcome trials. Nonetheless, it is of interest that Roush et al.,137 who compared the results of all the studies we summarize above in which the tested active hypertension medication is systematically ingested in the evening/bedtime with those from 170 clinical trials included in an earlier meta-analysis in which the investigated hypertension medication(s) is (are) ingested daily in the morning,138 report significant 48% better reduction (P=0.008) in relative risk of CVD events when hypertension medications are ingested at bedtime rather than morning.
The MAPEC study constitutes the first prospective trial specifically designed and conducted to completion to test the hypothesis that bedtime hypertension chronotherapy that focus specifically on the normalization of asleep BP mean and sleep-time relative BP decline better reduces CVD and stroke risk than conventional morning-time therapy.31, 32, 33, 37, 38, 39, 43, 44, 45, 46, 47 After a median follow-up of 5.6 years, hypertensive patients randomized to ingest the entire daily dose of ⩾1 BP-lowering medications at bedtime, in comparison with those randomized to ingest all prescribed hypertension medications upon awakening, display as expected—based upon the many previously conducted morning vs. bedtime treatment-time investigations59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69—significantly lower asleep BP mean, higher sleep-time relative BP decline, reduced prevalence of nondipping (34% vs. 62%; P<0.001) and higher prevalence of controlled ambulatory BP (62% vs. 53%, P<0.001). Most important, the bedtime therapy regimen, compared with the upon awakening one, results in significantly lower adjusted HR of total CVD events (HR=0.39, 95% CI 0.29–0.51, P<0.001) and major CVD events—composite of CVD death, myocardial infarction and ischemic and hemorrhagic stroke—(HR=0.33, 95% CI 0.19–0.55, P<0.001).43 Patients randomized to treatment upon awakening, no matter the class(es) of ingested BP-lowering medication(s), evidence highest CVD risk. Greater benefits are observed for bedtime than awakening treatment with ARBs (HR=0.29, 95% CI 0.17–0.51, P<0.001) and CCBs (HR=0.46, 95% CI 0.31–0.69, P<0.001);46 however, patients randomized to ingest at bedtime an ARB, in comparison with any other class of medication, with or without additional BP-lowering drugs, evidence significantly lowest HR of CVD events (P<0.017).46
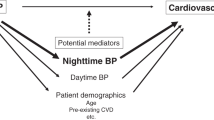
Thus, the MAPEC study not only substantiates asleep SBP mean to be the most significant and only independent BP-derived prognostic marker of CVD morbidity and mortality,31, 32, 37, 38 as earlier discussed, a finding also corroborated by several other prospective ABPM studies,22, 25, 26, 30, 34, 35, 36 but it also further substantiates that reduction of asleep SBP mean by a hypertension treatment strategy defined by ingestion of the entire daily dose of ⩾1 conventional BP-lowering medications at bedtime, especially when including an ARB, significantly and cost effectively decreases CVD risk, both for patients of the general hypertension population43 and those of greater vulnerability and enhanced CVD risk, that is, those diagnosed with type 2 diabetes,38, 44 CKD,45 and RH39 (Figure 6). In this regard, it is noteworthy that several international medical and scientific societies18, 41, 139, 140, 141, 142 now acknowledge the clinical relevance of this specific concept of hypertension chronotherapy by recommending physicians to advise their hypertensive patients to ingest ⩾1 of their prescribed BP-lowering medications at bedtime.
Kaplan–Meier survival curves for total cardiovascular disease (CVD) events as a function of time of day (with respect to circadian rhythms) of BP-lowering treatment, that is, for hypertensive patients ingesting either all prescribed BP-lowering medications upon awakening (continuous line) or full daily dose of ≥1 of them at bedtime (dashed line), in the general hypertension population of the (a) MAPEC (Monitorización Ambulatoria para Predicción de Eventos Cardiovasculares) study and those diagnosed upon recruitment with either (b) type 2 diabetes, (c) chronic kidney disease (CKD) or (d) resistant hypertension (RH) (updated from references 39, 43, 44, 45). A full color version of this figure is available at the Hypertension Research journal online.
Conclusions
The goal of all hypertension treatment strategies is reduction of SBP and DBP as a means of preventing end-organ injury and decreasing CVD, stroke, renal disease and other life-threatening risks. Early outcome trials report that prevention of CVD events by BP-lowering treatment is consistent and, to a certain extent, independent of class of prescribed medications. However, this finding is based primarily on outcome trials targeting correction of only the daytime OBPM level as opposed to correction of features—mainly asleep SBP mean and sleep-time relative SBP decline—of the 24 h BP pattern known to be more strongly associated with CVD risk. Current hypertension therapy strategies, almost exclusively focused upon attenuating daytime OBPM level,19 unfortunately have not effectively eliminated CVD hazards associated with elevated BP; they have only succeeded in moderating them by a suboptimal ~33%.143 Review of the many published OBPM-based outcome trials reveals that reduction of major CVD events to a relatively low level has been possible only in studies that specifically enrolled low-risk hypertensive patients, that is, ones without diabetes, CKD, previous CVD events, advanced organ injury and elderly.144 The collective findings of past studies involving high-risk patients managed by the conventional morning treatment strategy and aimed at attaining published thresholds for daytime OBPM reveal inability to sufficiently lower CVD and stroke morbidity and mortality risk.
The diagnostic approaches and treatment strategies that today dominate the clinical practice of medicine unfortunately disregard the facts that: (1) correlation between BP and CVD risk is far stronger for ABPM-derived asleep SBP mean and sleep-time relative SBP decline than daytime OBPM;22, 25, 26, 30, 31, 32, 34, 35, 36, 37, 38, 39, 40 and (2) BP-lowering efficacy and other beneficial effects on the daily BP pattern of six different classes of hypertension medications and their combinations exhibit statistically and clinically significant awakening vs. bedtime treatment differences.59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69 The findings of the MAPEC study, based upon periodic systematic 48 h ABPM evaluation of all participants during a median follow-up of 5.6 years, constitute the first proof-of-concept evidence that progressive reduction of the asleep SBP mean and correction of sleep-time relative SBP decline toward the normal dipper BP profile, most efficiently accomplished by a bedtime hypertension treatment strategy entailing conventional BP-lowering medications, best attenuates the risk of CVD, stroke and development of new-onset diabetes.31, 32, 33, 37, 38, 39, 43, 44, 45, 46, 47, 48, 49 These results document importance of 24 h BP control and pattern normalization, that is, specifically attenuation of sleep-time BP and increase of sleep-time BP relative decline, by bedtime conventional hypertension therapy, administered in step with key physiologic, neuroendocrine and other circadian rhythm determinants of the BP nyctohemeral pattern, as the best and most cost-effective means of achieving meaningful reduction of vascular and other hypertension-associated risks.
Future prospective long-term outcomes trials that incorporate periodic, annual or more frequent ABPM assessments and simultaneous diary recording of bed and wake times—to accurately and reliably ascertain asleep and wake-time BP level and dipping status—as done in the completed MAPEC study and currently ongoing Hygia Project,65, 107, 115, 116, 124, 125 are needed to confirm the highly significant independent prognostic value of sleep-time BP as well as the beneficial effects (reduced risk of CVD events and target tissue and organ injury) and safety of enhanced sleep-time BP reduction by bedtime hypertension chronotherapy with conventional medications. In the interim, we recommend this bedtime treatment strategy be adopted for management of individuals with predominant sleep-time hypertension or nondipper BP patterning as assessed by around-the-clock ABPM; this includes, but it is not limited to, the elderly and those diagnosed with elevated asleep BP because of diabetes, CKD, obstructive sleep apnea, history of past CVD events or resistance to pharmacotherapy intervention when timed upon awakening.18, 41, 42, 145
References
Duguay D, Cermakian N . The crosstalk between physiology and circadian clock proteins. Chronobiol Int 2009; 26: 1479–1513.
Reppert SM, Weaver DR . Coordination of circadian timing in mammals. Nature 2002; 418: 935e41.
Albrecht U . Timing to perfection: the biology of central and peripheral clocks. Neuron 2012; 74: 246e60.
Hermida RC, Fernández JR, Ayala DE, Mojón A, Alonso I, Smolensky M . Circadian rhythm of double (rate-pressure) product in healthy normotensive young subjects. Chronobiol Int 2001; 18: 475–489.
Hermida RC, Ayala DE, Fernández JR, Mojón A, Alonso I, Calvo C . Modeling the circadian variability of ambulatorily monitored blood pressure by multiple-component analysis. Chronobiol Int 2002; 19: 461–481.
Hermida RC, Calvo C, Ayala DE, Mojón A, López JE . Relationship between physical activity and blood pressure in dipper and nondipper hypertensive patients. J Hypertens 2002; 20: 1097–1104.
Portaluppi F, Vergnani L, Manfredini R, Fersini C . Endocrine mechanisms of blood pressure rhythms. Ann NY Acad Sci 1996; 783: 113–131.
Hermida RC, Ayala DE, Portaluppi F . Circadian variation of blood pressure: the basis for the chronotherapy of hypertension. Adv Drug Deliv Rev 2007; 59: 904–922.
Portaluppi F, Tiseo R, Smolensky MH, Hermida RC, Ayala DE, Fabbian F . Circadian rhythms and cardiovascular health. Sleep Med Rev 2012; 16: 151–166.
Fabbian F, Smolensky MH, Tiseo R, Pala M, Manfredini R, Portaluppi F . Dipper and non-dipper blood pressure 24-hour patterns: circadian rhythm-dependent physiologic and pathophysiologic mechanisms. Chronobiol Int 2013; 30: 17–30.
Lakatua DJ, Haus E, Halberg F, Halberg E, Wendt HW, Sackett-Lundeen LL, Berg HG, Kawasaki T, Ueno M, Uezono K, Matsuoka M, Omae T . Circadian characteristics of urinary epinephrine and norepinephrine from healthy young women in Japan and U.S.A. Chronobiol Int 1986; 3: 189–195.
Angeli A, Gatti G, Masera R . Chronobiology of the hypothalamic-pituitary-adrenal and renin-angiotensin-aldosterone systems. In: Touitou Y, Haus E (eds). Biologic Rhythms in Clinical and Laboratory Medicine. Springer-Verlag: Berlin. 1992, pp 292–314.
Kool MJ, Wijnen JA, Derkx FH, Struijker Boudier HA, Van Bortel LM . Diurnal variation in prorenin in relation to other humoral factors and hemodynamics. Am J Hypertens 1994; 7: 723–730.
Bartter FC, Chan JCM, Simpson HW . Chronobiological aspect of plasma renin activity, plasma aldosterone and urinary electrolytes. In: Krieger DT (ed). Endocrine Rhythms. Raven: New York. 1979 pp 49–132.
Portaluppi F, Trasforini G, Margutti A, Vergnani L, Ambrosio MR, Rossi R, Bagni B, Pansini R, degli Uberti EC . Circadian rhythm of calcitonin gene-related peptide in uncomplicated essential hypertension. J Hypertens 1992; 10: 1227–1234.
Sothern RB, Vesely DL, Kanabrocki EL, Hermida RC, Bremner FW, JLHC Third, Boles MA, Nemchausky BM, Olwin JH, Scheving LE . Temporal (circadian) and functional relationship between atrial natriuretic peptides and blood pressure. Chronobiol Int 1995; 12: 106–120.
Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F . Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med 2007; 8: 668–680.
Hermida RC, Smolensky MH, Ayala DE, Portaluppi F, Crespo JJ, Fabbian F, Haus E, Manfredini R, Mojón A, Moyá A, Piñeiro L, Ríos MT, Otero A, Balan H, Fernández JR . 2013 Ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals. Joint recommendations from the International Society for Chronobiology (ISC), American Association of Medical Chronobiology and Chronotherapeutics (AAMCC), Spanish Society of Applied Chronobiology, Chronotherapy, and Vascular Risk (SECAC), Spanish Society of Atherosclerosis (SEA), and Romanian Society of Internal Medicine (RSIM). Chronobiol Int 2013; 30: 355–410.
Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F . 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31: 1281–1357.
Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistell M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, Santucci C, Reboldi G . Ambulatory blood pressure: an independent predictor of prognosis in essential hypertension. Hypertension 1994; 24: 793–801.
Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E . Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med 2003; 348: 2407–2415.
Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E . Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension 2005; 46: 156–161.
Eguchi K, Pickering TG, Hoshide S, Ishikawa J, Ishikawa S, Schwartz J, Shimada K, Kario K . Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens 2008; 21: 443–450.
Salles GF, Cardoso CR, Muxfeldt ES . Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med 2008; 168: 2340–2346.
Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboni P, Conte G, Gabbai FB, De Nicola L . Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med 2011; 171: 1090–1098.
The ABC-H Investigators The ABC-H Investigators Roush GC, Fagard RH, Salles GF, Pierdomenico SD, Reboldi G, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida RC, Dolan E, Zamalloa H . Prognostic impact from clinic, daytime, and nighttime systolic blood pressure in 9 cohorts on 13,844 patients with hypertension. J Hypertens 2014; 32: 2332–2340.
Portaluppi F, Hermida RC . Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev 2007; 59: 940–951.
Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K . Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001; 38: 852–857.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y . Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189.
Boggia J, Li Y, Thijs L, Hansen TW, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Kuznetsova T, Torp-Pedersen CH, Lind L, Ibsen H, Imai Y, Wang J, Sandoya E, O’Brien E, Staessen JA . Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370: 1219–1229.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Decreasing sleep-time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol 2011; 58: 1165–1173.
Hermida RC, Ayala DE, Fernández JR, Mojón A . Sleep-time blood pressure: prognostic value and relevance as a therapeutic target for cardiovascular risk reduction. Chronobiol Int 2013; 30: 68–86.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level — the “normotensive non-dipper” paradox. Chronobiol Int 2013; 30: 87–98.
Kikuya M, Ohkubo T, Asayama K, Metoki H, Obara T, Saito S, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y . Ambulatory blood pressure and 10-year risk of cardiovascular and noncardiovascular mortality. The Ohasama Study. Hypertension 2005; 45: 240–245.
Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M . Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension 2007; 49: 1235–1241.
Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA . Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008; 51: 55–61.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Sleep-time blood pressure and the prognostic value of isolated-office and masked hypertension. Am J Hypertens. 2012; 25: 297–305.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Sleep-time blood pressure as a therapeutic target for cardiovascular risk reduction in type 2 diabetes. Am J Hypertens 2012; 25: 325–334.
Ayala DE, Hermida RC, Mojón A, Fernández JR . Cardiovascular risk of resistant hypertension: Dependence on treatment-time regimen of blood pressure-lowering medications. Chronobiol Int 2013; 30: 340–352.
Hermida RC, Ayala DE, Mojón A, Smolensky MH, Portaluppi F, Fernández JR . Sleep-time ambulatory blood pressure as a novel therapeutic target for cardiovascular risk reduction. J Hum Hypertens 2014; 28: 567–574.
Hermida RC, Smolensky MH, Ayala DE, Portaluppi F . Ambulatory blood pressure monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int (e-pub ahead of print 20 November 2015).
Smolensky MH, Ayala DE, Hermida RC . Ambulatory blood pressure monitoring (ABPM) as THE reference standard to confirm diagnosis of hypertension in adults: recommendation of the 2015 U.S. Preventive Services Task Force (USPSTF). Chronobiol Int 2015; 32: 1320–1322.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Influence of circadian time of hypertension treatment on cardiovascular risk: Results of the MAPEC study. Chronobiol Int 2010; 27: 1629–1651.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care 2011; 34: 1270–1276.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol 2011; 22: 2313–2321.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Cardiovascular risk of essential hypertension: Influence of class, number, and treatment-time regimen of hypertension medications. Chronobiol Int 2013; 30: 315–327.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Role of time-of-day of hypertension treatment on the J-shaped relationship between blood pressure and cardiovascular risk. Chronobiol Int 2013; 30: 328–339.
Hermida RC, Ayala DE, Mojón A, Fernández JR, Sleep-time BP . Prognostic marker of type 2 diabetes and therapeutic target for prevention. Diabetologia 2015 (in press) doi:10.1007/s00125-015-3748-8 [e-pub ahead of print].
Hermida RC, Ayala DE, Mojón A, Fernández JR . Bedtime ingestion of hypertension medications reduces the risk of new-onset type 2 diabetes: a randomised controlled trial. Diabetologia 2015 (in press) doi:10.1007/s00125-015-3749-7 [e-pub ahead of print].
Hermida RC, Ayala DE, Fontao MJ, Mojón A, Fernández JR . Ambulatory blood pressure monitoring: Importance of sampling rate and duration — 48 versus 24 hours — on the accurate assessment of cardiovascular risk. Chronobiol Int 2013; 30: 55–67.
Hermida RC, Ayala DE, Crespo JJ, Mojón A, Chayán L, Fontao MJ, Fernández JR . Influence of age and hypertension treatment-time on ambulatory blood pressure in hypertensive patients. Chronobiol Int 2013; 30: 176–191.
Hermida RC, Fernández JR, Mojón A, Ayala DE . Reproducibility of the hyperbaric index as a measure of blood pressure excess. Hypertension 2000; 35: 118–125.
Hermida RC, Mojón A, Fernández JR, Alonso I, Ayala DE . The tolerance-hyperbaric test: a chronobiologic approach for improved diagnosis of hypertension. Chronobiol Int 2002; 19: 1183–1211.
Crespo C, Aboy M, Fernández JR, Mojón A . Automatic identification of activity-rest periods based on actigraphy. Med Biol Eng Comput 2012; 50: 329–340.
Crespo C, Fernández JR, Aboy M, Mojón A . Clinical application of a novel automatic algorithm for actigraphy-based activity and rest period identification to accurately determine awake and asleep ambulatory blood pressure parameters and cardiovascular risk. Chronobiol Int 2013; 30: 43–54.
Bélanger PM, Bruguerolle B, Labrecque G . Rhythms in pharmacokinetics: absorption, distribution, metabolism. In: Redfern PH, Lemmer B (eds). Physiology and Pharmacology of Biological Rhythms. Handbook of Experimental Pharmacology series Vol 125. Springer-Verlag: Berlin-New York. 1997, pp 177–204.
Labrecque G, Beauchamp D . Rhythms and pharmacokinetics. In: Redfern P (ed). Chronotherapeutics. Pharmaceutical Press: London. 2003, pp 75–110.
Okyar A, Dressler C, Hanafy A, Baktir G, Lemmer B, Spahn-Langguth H . Circadian variations in exsorptive transport: in-situ intestinal perfusion data and in-vivo relevance. Chronobiol Int 2012; 29: 443–453.
Hermida RC, Ayala DE, Calvo C, Portaluppi F, Smolensky MH . Chronotherapy of hypertension: administration-time dependent effects of treatment on the circadian pattern of blood pressure. Adv Drug Deliv Rev 2007; 59: 923–939.
Smolensky MH, Hermida RC, Ayala DE, Tiseo R, Portaluppi F . Administration-time-dependent effect of blood pressure-lowering medications: basis for the chronotherapy of hypertension. Blood Press Monit 2010; 15: 173–180.
Hermida RC, Ayala DE, Fernández JR, Portaluppi F, Fabbian F, Smolensky MH . Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens 2011; 24: 383–391.
Smolensky MH, Siegel RA, Haus E, Hermida RC, Portaluppi F . Biological rhythms, drug delivery, and chronotherapeutics In Siepmann J, Siegel RA, Rathbone MJ (eds). Fundamentals and Applications of Controlled Release Drug Delivery. Springer-Verlag: Heildelberg. 2012, pp 359–443.
Hermida RC, Ayala DE, Fernández JR, Mojón A, Smolensky MH, Fabbian F, Portaluppi F . Administration-time-differences in effects of hypertension medications on ambulatory blood pressure regulation. Chronobiol Int 2013; 30: 280–314.
Hermida RC, Ayala DE, Smolensky MH, Mojón A, Fernández JR, Crespo JJ, Moyá A, Ríos MT, Portaluppi F . Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol 2013; 9: 358–368.
Hermida RC, Ríos MT, Crespo JJ, Moyá A, Domínguez-Sardiña M, Otero A, Sánchez JJ, Mojón A, Fernández JR, Ayala DE . on behalf on the Hygia Project Investigators. Treatment-time regimen of hypertension medications significantly affects ambulatory blood pressure and clinical characteristics of patients with resistant hypertension. Chronobiol Int 2013; 30: 192–206.
Hermida RC, Ayala DE, Smolensky MH, Fernández JR, Mojón A, Crespo JJ, Ríos MT, Moyá A, Portaluppi F . Chronotherapeutics of conventional blood pressure-lowering medications: simple, low-cost means of improving management and treatment outcomes of hypertensive-related disorders. Curr Hypertens Rep 2014; 16: 412.
Hermida RC, Ayala DE, Smolensky MH, Mojón A, Fernández JR, Portaluppi F . Chronotherapy of hypertension with ACEIs and CKD — a new solution to an old problem. In: Onuigbo M (ed). ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics Vol 2. Nova Science Publishers Inc: Hauppauge, NY. 2014, pp 3–39.
Hermida RC, Smolensky MH, Ayala DE, Fernández JR, Moyá A, Crespo JJ, Mojón A, Ríos MT, Fabbian F, Portaluppi F . Abnormalities in chronic kidney disease of ambulatory blood pressure 24h patterning and normalization by bedtime hypertension chronotherapy. Nephrol Dial Transplant 2014; 29: 1160–1167.
Smolensky MH, Hermida RC, Ayala DE, Portaluppi F . Bedtime hypertension chronotherapy: concepts and patient outcomes. Curr Pharm Des 2015; 21: 773–790.
Witte K, Lemmer B . Rhythms and pharmacodynamics. In: Redfern P (ed). Chronotherapeutics. Pharmaceutical Press: London. 2003, pp 111–126.
Hermida RC, Ayala DE . Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension 2009; 54: 40–46.
Hermida RC, Ayala DE, Fontao MJ, Mojón A, Alonso I, Fernández JR . Administration-time-dependent effects of spirapril on ambulatory blood pressure in uncomplicated essential hypertension. Chronobiol Int 2010; 27: 560–574.
Balan H, Popescu E, Angelescu G . Comparing different treatment schedules of Zomen (zofenopril). Rom J Intern Med 2011; 49: 75–84.
Ohmori M, Fujimura A . ACE inhibitors and chronotherapy. Clin Exp Hypertens 2005; 2 & 3: 179–185.
Sunaga K, Fujimura A, Shiga T, Ebihara A . Chronopharmacology of enalapril in hypertensive patients. Eur J Clin Pharmacol 1995; 48: 441–445.
Fujimura A, Ebihara A, Shiigai T, Shimada K, Tagawa H, Gomi T, Nakamura Y, Suzuki M, Yokozuka H . Amelioration of enalapril-induced dry cough by changing dosing time from morning to evening: a preliminary trial. Jpn J. Clin Pharmacol Ther 1999; 30: 741–744.
Pechère-Bertschi A, Nussberger J, Decosterd L, Armagnac C, Sissmann J, Bouroudian M, Brunner HR, Burnier M . Renal response to the angiotensin II receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J Hypertens 1988; 16: 385–393.
Hermida RC, Calvo C, Ayala DE, Domínguez MJ, Covelo M, Fernández JR, Mojón A, López JE . Administration-time-dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension 2003; 42: 283–290.
Hermida RC, Calvo C, Ayala DE, Fernández JR, Covelo M, Mojón A, López JE . Treatment of non-dipper hypertension with bedtime administration of valsartan. J Hypertens 2005; 23: 1913–1922.
Hermida RC, Calvo C, Ayala DE, López JE . Decrease in urinary albumin excretion associated to the normalization of nocturnal blood pressure in hypertensive subjects. Hypertension 2005; 46: 960–968.
Hermida RC, Calvo C, Ayala DE, Mojón A, Rodríguez M, Chayán L, López JE, Fontao MJ, Soler R, Fernández JR . Administration time-dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int 2005; 22: 755–776.
Hermida RC, Ayala DE, Calvo C . Optimal timing of antihypertensive dosing: focus on valsartan. Ther Clin Risk Manag 2007; 3: 119–131.
Hermida RC, Ayala DE, Fernández JR, Calvo C . Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 2007; 50: 715–722.
Hermida RC, Ayala DE, Chayán L, Mojón A, Fernández JR . Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int 2009; 26: 61–79.
Kario K, Hoshide S, Shimizu M, Yano Y, Eguchi K, Ishikawa J, Ishikawa S, Shimada K . Effects of dosing time of angiotensin II receptor blockade titrated by self-measured blood pressure recordings on cardiorenal protection in hypertensives: the Japan Morning Surge-Target Organ Protection (J-TOP) study. J Hypertens 2010; 28: 1574–1583.
Eguchi K, Shimizu M, Hoshide S, Shimada K, Kario K . A bedtime dose of ARB was better than a morning dose in improving baroreflex sensitivity and urinary albumin excretion—the J-TOP study. Clin Exp Hypertens 2012; 34: 488–492.
Hoshino A, Nakamura T, Matsubara H . The bedtime administration ameliorates blood pressure variability and reduces urinary albumin excretion in amlodipine-olmesartan combination therapy. Clin Exp Hypertens 2010; 32: 416–422.
Kasiakogias A, Tsioufis C, Thomopoulos C, Andrikou I, Aragiannis D, Dimitriadis K, Tsiachris D, Bilo G, Sideris S, Filis K, Parati G, Stefanadis C . Evening versus morning dosing of antihypertensive drugs in hypertensive patients with sleep apnoea: a cross-over study. J Hypertens 2015; 33: 393–400.
Zappe DH, Crikelair N, Kandra A, Palatini P . Time of administration important? Morning versus evening dosing of valsartan. J Hypertens 2015; 33: 385–392.
Calvo C, Hermida RC, Ayala DE, Ruilope LM . Effects of telmisartan 80 mg and valsartan 160 mg on ambulatory blood pressure in patients with essential hypertension. J Hypertens 2004; 22: 837–846.
Hermida RC, Calvo C, Ayala DE, Lopez JE, Rodriguez M, Chayan L, Mojon A, Fontao MJ, Fernandez JR . Dose- and administration-time-dependent effects of nifedipine GITS on ambulatory blood pressure in hypertensive subjects. Chronobiol Int 2007; 24: 471–493.
Hermida RC, Ayala DE, Mojon A, Fernandez JR . Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. Am J Hypertens 2008; 21: 948–954.
Hermida RC, Calvo C, Ayala DE, Domínguez MJ, Covelo M, Fernández JR, Fontao MJ, López JE . Administration-time-dependent effects of doxazosin GITS on ambulatory blood pressure of hypertensive subjects. Chronobiol Int 2004; 21: 277–296.
Koga H, Hayashi J, Yamamoto M, Kitamoto K . Prevention of morning surge of hypertension by the evening administration of carvedilol. Jap Med Ass J 2005; 48: 398–403.
Hermida RC, Calvo C, Ayala DE, Rodríguez M, Chayán L, López JE . Administration time-dependent effects of nebivolol on the diurnal/nocturnal blood pressure ratio in hypertensive patients. J Hypertens 2006; 24 (Suppl 4): S89.
Hermida RC, Ayala DE, Mojón A, Chayán L, Domínguez MJ, Fontao MJ, Soler R, Alonso I, Fernández JR . Comparison of the effects on ambulatory blood pressure of awakening versus bedtime administration of torasemide in essential hypertension. Chronobiol Int 2008; 25: 950–970.
Middeke M, Kluglich M, Holzgreve H . Chronopharmacology of captopril plus hydrochlorothiazide in hypertension: morning versus evening dosing. Chronobiol Int 1991; 8: 506–510.
Meng Y, Zhang Z, Liang X, Wu C, Qi G . Effects of combination therapy with amlodipine and fosinopril administered at different times on blood pressure and circadian blood pressure pattern in patients with essential hypertension. Acta Cardiol 2010; 65: 309–314.
Zeng J, Jia M, Ran H, Zhang Y, Zhang J, Wang X, Wang H, Yang C, Zeng C . Fixed-combination of amlodipine and diuretic chronotherapy in the treatment of essential hypertension: improved blood pressure control with bedtime dosing—a multicenter, open-label randomized study. Hypertens Res 2011; 34: 767–772.
Hermida RC, Ayala DE, Fontao MJ, Mojón A, Fernández JR . Chronotherapy with valsartan/amlodipine combination in essential hypertension: improved blood pressure control with bedtime dosing. Chronobiol Int 2010; 27: 1287–1303.
Hermida RC, Ayala DE, Mojón A, Fontao MJ, Fernández JR . Chronotherapy with valsartan/hydrochlorothiazide combination in essential hypertension: improved sleep-time blood pressure control with bedtime dosing. Chronobiol Int 2011; 28: 601–610.
Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM . Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51: 1403–1419.
Cugini P . The treatability of refractory or resistant hypertension by personalized antihypertensive chronotherapy based on ambulatory monitoring of the arterial pressure. Recenti Prog Med 1996; 87: 51–57.
Hermida RC, Ayala DE, Calvo C, López JE, Mojón A, Fontao MJ, Soler R, Fernández JR . Effects of the time of day of antihypertensive treatment on the ambulatory blood pressure pattern of patients with resistant hypertension. Hypertension 2005; 46: 1053–1059.
Hermida RC, Ayala DE, Fernández JR, Calvo C . Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 2008; 51: 69–76.
Hermida RC, Ayala DE, Mojón A, Fernández JR . Effects of time of antihypertensive treatment on ambulatory blood pressure and clinical characteristics of subjects with resistant hypertension. Am J Hypertens 2010; 23: 432–439.
Ríos MT, Domínguez-Sardiña M, Ayala DE, Gomara S, Sineiro E, Pousa L, Callejas PA, Fontao MJ, Fernández JR, Hermida RC, on behalf of the Hygia Project Investigators. Prevalence and clinical characteristics of isolated-office and true resistant hypertension determined by ambulatory blood pressure monitoring. Chronobiol Int 2013; 30: 207–220.
Almirall J, Comas L, Martínez-Ocaña JC, Roca S, Arnau A . Effects of chronotherapy on blood pressure control in non-dipper patients with refractory hypertension. Nephrol Dial Transplant 2012; 27: 1855–1859.
Niskikawa T, Omura M, Saito J, Matsuzawa Y . The possibility of resistant hypertension during the treatment of hypertensive patients. Hypertens Res 2013; 36: 924–929.
Agarwal R, Nissenson AR, Battle D, Coyne DW, Trout JR, Warnock DG . Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 2003; 115: 291–297.
Portaluppi F, Montanari L, Ferlini M, Gilli P . Altered circadian rhythms of blood pressure and heart rate in non-hemodialysis chronic renal failure. Chronobiol Int 1990; 7: 321–327.
Agarwal R, Andersen MJ . Correlates of systolic hypertension in patients with chronic kidney disease. Hypertension 2005; 46: 514–520.
Davidson MB, Hix JK, Vidt DG, Brotman DJ . Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med 2006; 166: 846–852.
Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, Hu B, Greene T, Appel L, Phillips RA, African American Study of Kidney Disease and Hypertension Collaborative Research Group. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 2009; 53: 20–27.
Crespo JJ, Piñeiro L, Otero A, Castiñeira C, Ríos MT, Regueiro A, Mojón A, Lorenzo S, Ayala DE, Hermida RC, on behalf of the Hygia Project Investigators. Administration-time-dependent effects of hypertension treatment on ambulatory blood pressure in patients with chronic kidney disease. Chronobiol Int 2013; 30: 159–175.
Mojón A, Ayala DE, Piñeiro L, Otero A, Crespo JJ, Moyá A, Bóveda J, Pérez de Lis J, Fernández JR, Hermida RC, on behalf of the Hygia Project Investigators. Comparison of ambulatory blood pressure parameters of hypertensive patients with and without chronic kidney disease. Chronobiol Int 2013; 30: 145–158.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluaion and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150.
Minutolo R, Gabbai FB, Borrelli S, Scigliano R, Trucillo P, Baldanza D, Laurino S, Mascia S, Conte G, De Nicola L . Changing the timing of antihypertensive therapy to reduce nocturnal blood pressure in CKD: an 8-week uncontrolled trial. Am J Kidney Dis 2007; 50: 908–917.
Rahman M, Greene T, Phillips RA, Agodoa LY, Bakris GL, Charleston J, Contreras G, Gabbai F, Hiremath L, Jamerson K, Kendrick C, Kusek JW, Lash JP, Lea J, Miller ER 3rd, Rostand S, Toto R, Wang X, Wright JT Jr, Appel LJ . A trial of 2 strategies to reduce nocturnal blood pressure in blacks with chronic kidney disease. Hypertension 2013; 61: 82–88.
Okeahialam B, Ohihoin E, Ajuluchukwu J . Chronotherapy in Nigerian hypertensives. Ther Adv Cardiovasc Dis 2011; 5: 113–118.
Wang C, Zhang J, Liu X, Li CC, Ye ZC, Peng H, Chen Z, Lou T . Effect of valsartan with bedtime dosing on chronic kidney disease patients with nondipping blood pressure pattern. J Clin Hypertens (Greenwich) 2013; 15: 48–54.
Cuspidi C, Meani S, Lonati L, Fusi V, Valerio C, Sala C, Magnaghi G, Maisaidi M, Zanchetti A . Short-term reproducibility of a non-dipping pattern in type 2 diabetic hypertensive patients. J Hypertens 2006; 24: 647–653.
Pistrosch F, Reissmann E, Wildbrett J, Koehler C, Hanefeld M . Relationship between diurnal blood pressure variation and diurnal blood glucose levels in type 2 diabetic patients. Am J Hypertens 2007; 20: 541–545.
Ayala DE, Moyá A, Crespo JJ, Castiñeira C, Domínguez-Sardiña M, Gomara S, Sineiro E, Mojón A, Fontao MJ, Hermida RC, on behalf of the Hygia Project Investigators. Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes. Chronobiol Int 2013; 30: 99–115.
Moyá A, Crespo JJ, Ayala DE, Ríos MT, Pousa L, Callejas PA, Salgado JL, Mojón A, Fernández JR, Hermida RC, on behalf on the Hygia Project Investigators. Effects of time-of-day of hypertension treatment on ambulatory blood pressure and clinical characteristics of patients with type 2 diabetes. Chronobiol Int 2013; 30: 116–131.
Tofé S, García B . 24-hour and nighttime blood pressures in type 2 diabetic hypertensive patients following morning or evening administration of olmesartan. J Clin Hypertens (Greenwhich) 2009; 11: 426–431.
Rossen NB, Knudsen ST, Fleischer J, Hvas AM, Ebbehoj, Poulsen PL, Hansen KW . Targetting nocturnal hyperetnsion in type 2 diabetes mellitus. Hypertension 2014; 64: 1080–1087.
Suzuki K, Aizawa Y . Evaluation of dosing time-related anti-hypertensive efficacy of valsartan in patients with type 2 diabetes. Clin Exp Hypertens 2011; 33: 56–62.
Qiu YG, Zhu JH, Tao QM, Zheng P, Chen JZ, Hu SJ, Zhang FR, Zheng LR, Zhao LL, Yao XY . Captopril administered at night restores the diurnal blood pressure rhythm in adequately controlled, nondipping hypertensives. Cardiovasc Drugs Ther 2005; 19: 189–195.
Takeda A, Toda T, Fujii T, Matsui N . Bedtime administration of long-acting antihypertensive drugs restores normal nocturnal blood pressure fall in nondippers with essential hypertension. Clin Exp Nephrol 2009; 13: 467–472.
Farah R, Makhoul N, Arraf Z, Khamisy-Farah R . Switching therapy to bedtime for uncontrolled hypertension with a nondipping pattern: a prospective randomized-controlled study. Blood Press Monit 2013; 18: 227–231.
Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G . Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000; 342: 145–153.
Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhager WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A . Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. Lancet 1997; 350: 757–764.
Liu L, Wang JG, Gong L, Liu G, Staessen JA . Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens 1998; 16: 1823–1829.
Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH Jr, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A, Anders RJ, CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 2003; 289: 2073–2082.
White WB, Black HR, Weber MA, Elliott WJ, Brysinski B, Fakourhi TD . Comparison of effects of controlled-onset extended-release verapamil at bedtime and nifedipine gastrointestinal therapeutic system on arising on early morning blood pressure, heart rate, and the heart rate-blood pressure product. Am J Cardiol 1998; 81: 424–431.
Roush GC, Fapohunda J, Kostis JB . Evening dosing of antihypertensive therapy to reduce cardiovascular events: a third type of evidence based on a systematic review and meta-analysis of randomized trials. J Clin Hypertens (Greenwich) 2014; 16: 561–568.
Law MR, Morris JK, Wald NJ . Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338: b1665.
American Diabetes Association. Standards of medical care in diabetes – 2012. Diabetes Care 2012; 35 (Suppl 1): S11–S63.
The Task Force of diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and European Association for the Study of Diabetes (EASD) The Task Force of diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and European Association for the Study of Diabetes (EASD) Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL . ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2013; 34: 3035–3087.
Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37: 253–390.
Chiang CE, Wang TD, Ueng KC, Lin TH, Yeh HI, Chen CY, Wu YJ, Tsai WC, Chao TH, Chen CH, Chu PH, Chao CL, Liu PY, Sung SH, Cheng HM, Wang KL, Li YH, Chiang FT, Chen JH, Chen WJ, Yeh SJ, Lin SJ 2015 guidelines of the Taiwan Society of Cardiology and the Taiwan Hypertension Society for the Management of Hypertension. J Chin Med Assoc 2015; 78: 1–47.
Gradman AH . Sleep-time blood pressure. A validated therapeutic target. J Am Coll Cardiol 2011; 58: 1174–1175.
Zanchetti A . Bottom blood pressure or bottom cardiovascular risk? How far can cardiovascular risk be reduced? J Hypertens 2009; 27: 1509–1520.
Portaluppi F, Smolensky MH . Perspectives on the chronotherapy of hypertension based on the results of the MAPEC study. Chronobiol Int 2010; 27: 1652–1667.
Acknowledgements
This research was supported by unrestricted grants from Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Spanish Government (PI14-00205); Ministerio de Ciencia e Innovación, Spanish Government (SAF2006-6254-FEDER; SAF2009-7028-FEDER); Consellería de Economía e Industria, Xunta de Galicia (09CSA018322PR); European Research Development Fund and Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (CN2012/251; CN2012/260; GPC2014/078); Atlantic Research Center for Information and Communication Technologies (AtlantTIC); and Vicerrectorado de Investigación, University of Vigo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hermida, R., Ayala, D., Smolensky, M. et al. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res 39, 277–292 (2016). https://doi.org/10.1038/hr.2015.142
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2015.142
Keywords
This article is cited by
-
Effectiveness of renal denervation in the treatment of hypertension: a literature review
Clinical Hypertension (2022)
-
Effect of CPAP treatment on BP in resistant hypertensive patients according to the BP dipping pattern and the presence of nocturnal hypertension
Hypertension Research (2022)
-
Does Timing of Antihypertensive Medication Dosing Matter?
Current Cardiology Reports (2020)
-
On the use of actigraphy in clinical evaluation of diurnal blood pressure profile
Somnologie (2020)
-
Treatment patterns and adherence to antihypertensive combination therapies in Japan using a claims database
Hypertension Research (2019)